Articles
- Page Path
- HOME > Ann Occup Environ Med > Volume 36; 2024 > Article
- Original Article Association between serum perfluoroalkyl substances concentrations and non-alcoholic fatty liver disease among Korean adults: a cross-sectional study using the National Environmental Health Survey cycle 4
-
Yong Tae Park
 , Eui Yup Chung
, Eui Yup Chung , Chang Ho Chae
, Chang Ho Chae , Young Hoon Lee
, Young Hoon Lee
-
Annals of Occupational and Environmental Medicine 2024;36:e10.
DOI: https://doi.org/10.35371/aoem.2024.36.e10
Published online: April 8, 2024
Department of Occupational and Environmental Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- Correspondence: Eui Yup Chung. Department of Occupational and Environmental Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, 158 Paryong-ro, Masanhoewon-gu, Changwon 51353, Korea. oemeuiyup@daum.net
Copyright © 2024 Korean Society of Occupational & Environmental Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background Perfluoroalkyl substances (PFAS) are widely used in industry and daily life due to their useful properties. They have a long half-life, accumulate in the body, and there is evidence that they are associated with biomarkers of lipid metabolism and liver damage. This may suggest non-alcoholic fatty liver disease (NAFLD) caused by PFAS. However, since there has been no study analyzing the relationship between PFAS and NAFLD in the entire population in Korea. We sought to confirm the relationship between serum PFAS concentration and NAFLD prevalence in Korean adults using the Korean National Environmental Health Survey (KoNEHS) cycle 4.
-
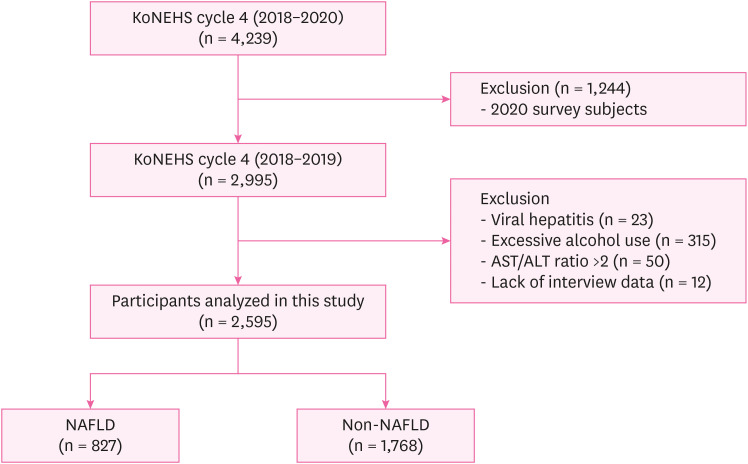
Methods The study was conducted on 2,529 subjects in 2018–2019 among KoNEHS participants. For the diagnosis of NAFLD, the hepatic steatosis index (HSI) was used, and the geometric mean and concentration distribution of serum PFAS were presented. Logistic regression was performed to confirm the increase in the risk of NAFLD due to changes in PFAS concentration, and the odds ratio and 95% confidence interval (CI) were calculated.
-
Results In both adjusted and unadjusted models, an increased odds ratio was observed with increasing serum concentrations of total PFAS and perfluorooctane sulfonate (PFOS) in the non-obese group. In the adjusted model, the odds ratios for serum total PFAS and PFOS were 6.401 (95% CI: 1.883–21.758) and 7.018 (95% CI: 2.688–18.319).
-
Conclusions In this study, a higher risk of NAFLD based on HSI was associated with serum total PFAS, PFOS in non-obese group. Further research based on radiological or histological evidence for NAFLD diagnosis and long-term prospective studies are necessary. Accordingly, it is necessary to find ways to reduce exposure to PFAS in industry and daily life.
BACKGROUND
METHODS
Selection of analytic samples in this study.
RESULTS
General characteristics of the study population according to NAFLD
Distribution of serum PFAS concentrations (μg/L) according to NAFLD status, sex, age group and BMI
The risk of NAFLD by PFAS concentration
The risk of NAFLD by PFAS concentration to stratified on BMI
DISCUSSION
CONCLUSIONS
Acknowledgements
Abbreviations
ALT
AST
BMI
CI
DM
EDC
FFA
GGT
HSI
IRB
KoNEHS
LDL-C
NAFLD
OR
PFAS
PFDeA
PFHxS
PFNA
PFOA
PFOS
PPAR
TG
-
Competing interests: The authors declare that they have no competing interests.
-
Author contributions:
Conceptualization: Park YT.
Data curation: Chung EY, Lee YH.
Formal analysis: Park YT.
Funding acquisition: Chung EY.
Investigation: Park YT, Lee YH.
Methodology: Chung EY, Chae CH.
Software: Park YT.
Validation: Chung EY, Chae CH.
Visualization: Park YT.
Writing - original draft: Park YT.
Writing - review & editing: Park YT, Chung EY, Chae CH.
NOTES
- 1. European Association for the Study of the Liver (EASL). European Association for the Study of Diabetes (EASD). European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64(6):1388–1402. 27062661.PubMed
- 2. Fang YL, Chen H, Wang CL, Liang L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: from “two hit theory” to “multiple hit model”. World J Gastroenterol 2018;24(27):2974–2983. 30038464.ArticlePubMedPMC
- 3. Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med 2017;377(21):2063–2072. 29166236.ArticlePubMed
- 4. Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2021;18(4):223–238. 33349658.ArticlePubMedPDF
- 5. Kang SY, Kim YJ, Park HS. Trends in the prevalence of non-alcoholic fatty liver disease and its future predictions in Korean men, 1998–2035. J Clin Med 2020;9(8):2626. 32823604.ArticlePubMedPMC
- 6. Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol 2017;67(4):862–873. 28642059.ArticlePubMed
- 7. Foulds CE, Treviño LS, York B, Walker CL. Endocrine-disrupting chemicals and fatty liver disease. Nat Rev Endocrinol 2017;13(8):445–457. 28524171.ArticlePubMedPMCPDF
- 8. Cano R, Pérez JL, Dávila LA, Ortega Á, Gómez Y, Valero-Cedeño NJ, et al. Role of endocrine-disrupting chemicals in the pathogenesis of non-alcoholic fatty liver disease: a comprehensive review. Int J Mol Sci 2021;22(9):4807. 34062716.ArticlePubMedPMC
- 9. Kim SK, Im JK, Kang YM, Jung SY, Kho YL, Zoh KD. Wastewater treatment plants (WWTPs)-derived national discharge loads of perfluorinated compounds (PFCs). J Hazard Mater 2012;201-202:82–91. 22169145.ArticlePubMed
- 10. Park H, Choo G, Kim H, Oh JE. Evaluation of the current contamination status of PFASs and OPFRs in South Korean tap water associated with its origin. Sci Total Environ 2018;634:1505–1512. 29710648.ArticlePubMed
- 11. CompTox Chemicals Dashboard. Updated 2022]. Accessed October 24, 2023]. https://comptox.epa.gov/dashboard/chemical-lists/PFASSTRUCTV5 .
- 12. Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 2019;29(2):131–147. 30470793.ArticlePubMedPMCPDF
- 13. Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 2007;115(9):1298–1305. 17805419.ArticlePubMedPMC
- 14. Zhang B, He Y, Huang Y, Hong D, Yao Y, Wang L, et al. Novel and legacy poly- and perfluoroalkyl substances (PFASs) in indoor dust from urban, industrial, and e-waste dismantling areas: the emergence of PFAS alternatives in China. Environ Pollut 2020;263(Pt A):114461. 32251969.ArticlePubMed
- 15. Fenton SE, Ducatman A, Boobis A, DeWitt JC, Lau C, Ng C, et al. Per- and polyfluoroalkyl substance toxicity and human health review: current state of knowledge and strategies for informing future research. Environ Toxicol Chem 2021;40(3):606–630. 33017053.ArticlePubMedPDF
- 16. Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 2007;99(2):366–394. 17519394.ArticlePubMed
- 17. Zeng XW, Qian Z, Emo B, Vaughn M, Bao J, Qin XD, et al. Association of polyfluoroalkyl chemical exposure with serum lipids in children. Sci Total Environ 2015;512-513:364–370. 25638651.ArticlePubMed
- 18. Dong Z, Wang H, Yu YY, Li YB, Naidu R, Liu Y. Using 2003–2014 U.S. NHANES data to determine the associations between per- and polyfluoroalkyl substances and cholesterol: trend and implications. Ecotoxicol Environ Saf 2019;173:461–468. 30798190.ArticlePubMed
- 19. Nemes K, Åberg F, Gylling H, Isoniemi H. Cholesterol metabolism in cholestatic liver disease and liver transplantation: from molecular mechanisms to clinical implications. World J Hepatol 2016;8(22):924–932. 27574546.ArticlePubMedPMC
- 20. Pratt DS. Evaluation of liver function. In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, editors. Harrison's Principles of Internal Medicine. 20th ed. New York, NY, USA: McGraw-Hill Education; 2018.
- 21. Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis 2010;42(7):503–508. 19766548.ArticlePubMed
- 22. Lee YH, Bang H, Park YM, Bae JC, Lee BW, Kang ES, et al. Non-laboratory-based self-assessment screening score for non-alcoholic fatty liver disease: development, validation and comparison with other scores. PLoS One 2014;9(9):e107584. 25216184.ArticlePubMedPMC
- 23. Feng C, Wang H, Lu N, Chen T, He H, Lu Y, et al. Log-transformation and its implications for data analysis. Shanghai Jingshen Yixue 2014;26(2):105–109.
- 24. Jain RB, Ducatman A. Selective associations of recent low concentrations of perfluoroalkyl substances with liver function biomarkers: NHANES 2011 to 2014 data on US adults aged ≥20 years. J Occup Environ Med 2019;61(4):293–302. 30589657.PubMed
- 25. E L. Zhang S, Jiang X. Association between perfluoroalkyl substances exposure and the prevalence of nonalcoholic fatty liver disease in the different sexes: a study from the National Health and Nutrition Examination Survey 2005–2018. Environ Sci Pollut Res Int 2023;30(15):44292–44303. 36692718.PubMed
- 26. Borghese MM, Liang CL, Owen J, Fisher M. Individual and mixture associations of perfluoroalkyl substances on liver function biomarkers in the Canadian Health Measures Survey. Environ Health 2022;21(1):85. 36104725.ArticlePubMedPMCPDF
- 27. Kim OJ, Kim S, Park EY, Oh JK, Jung SK, Park S, et al. Exposure to serum perfluoroalkyl substances and biomarkers of liver function: the Korean national environmental health survey 2015–2017. Chemosphere 2023;322:138208. 36822523.ArticlePubMed
- 28. Nian M, Li QQ, Bloom M, Qian ZM, Syberg KM, Vaughn MG, et al. Liver function biomarkers disorder is associated with exposure to perfluoroalkyl acids in adults: isomers of C8 health project in China. Environ Res 2019;172:81–88. 30776734.ArticlePubMed
- 29. Sohn JH, Kim TY. Recent update on pathogenesis of nonalcoholic fatty liver disease. Korean J Med 2010;79(5):461–474.
- 30. Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 2007;45(6):1366–1374. 17476695.ArticlePubMed
- 31. Cave MC, Clair HB, Hardesty JE, Falkner KC, Feng W, Clark BJ, et al. Nuclear receptors and nonalcoholic fatty liver disease. Biochim Biophys Acta 2016;1859(9):1083–1099. 26962021.ArticlePubMedPMC
- 32. Kersten S. Integrated physiology and systems biology of PPARα. Mol Metab 2014;3(4):354–371. 24944896.ArticlePubMedPMC
- 33. Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, et al. PPAR γ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 1999;4(4):611–617. 10549292.ArticlePubMed
- 34. Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-α, -β, and -γ, liver X receptor-β, and retinoid X receptor-α. Toxicol Sci 2006;92(2):476–489. 16731579.ArticlePubMed
- 35. Kirkley AG, Sargis RM. Environmental endocrine disruption of energy metabolism and cardiovascular risk. Curr Diab Rep 2014;14(6):494. 24756343.ArticlePubMedPMCPDF
- 36. Sheng N, Cui R, Wang J, Guo Y, Wang J, Dai J. Cytotoxicity of novel fluorinated alternatives to long-chain perfluoroalkyl substances to human liver cell line and their binding capacity to human liver fatty acid binding protein. Arch Toxicol 2018;92(1):359–369. 28864880.ArticlePubMedPDF
- 37. Genuis SJ, Birkholz D, Ralitsch M, Thibault N. Human detoxification of perfluorinated compounds. Public Health 2010;124(7):367–375. 20621793.ArticlePubMed
- 38. Fletcher T, Galloway TS, Melzer D, Holcroft P, Cipelli R, Pilling LC, et al. Associations between PFOA, PFOS and changes in the expression of genes involved in cholesterol metabolism in humans. Environ Int 2013;57-58:2–10. 23624243.ArticlePubMed
- 39. Yang W, Ling X, He S, Cui H, Yang Z, An H, et al. PPARα/ACOX1 as a novel target for hepatic lipid metabolism disorders induced by per- and polyfluoroalkyl substances: an integrated approach. Environ Int 2023;178:108138. 37572494.ArticlePubMed
- 40. Schlezinger JJ, Puckett H, Oliver J, Nielsen G, Heiger-Bernays W, Webster TF. Perfluorooctanoic acid activates multiple nuclear receptor pathways and skews expression of genes regulating cholesterol homeostasis in liver of humanized PPARα mice fed an American diet. Toxicol Appl Pharmacol 2020;405:115204. 32822737.ArticlePubMedPMC
- 41. Wan C, Han R, Liu L, Zhang F, Li F, Xiang M, et al. Role of miR-155 in fluorooctane sulfonate-induced oxidative hepatic damage via the Nrf2-dependent pathway. Toxicol Appl Pharmacol 2016;295:85–93. 26844784.ArticlePubMed
- 42. Fang X, Zou S, Zhao Y, Cui R, Zhang W, Hu J, et al. Kupffer cells suppress perfluorononanoic acid-induced hepatic peroxisome proliferator-activated receptor α expression by releasing cytokines. Arch Toxicol 2012;86(10):1515–1525. 22648072.ArticlePubMedPDF
- 43. Gallo V, Leonardi G, Genser B, Lopez-Espinosa MJ, Frisbee SJ, Karlsson L, et al. Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population with elevated PFOA exposure. Environ Health Perspect 2012;120(5):655–660. 22289616.ArticlePubMedPMC
- 44. Jin R, McConnell R, Catherine C, Xu S, Walker DI, Stratakis N, et al. Perfluoroalkyl substances and severity of nonalcoholic fatty liver in Children: an untargeted metabolomics approach. Environ Int 2020;134:105220. 31744629.ArticlePubMed
- 45. Costello E, Rock S, Stratakis N, Eckel SP, Walker DI, Valvi D, et al. Exposure to per- and polyfluoroalkyl substances and markers of liver injury: a systematic review and meta-analysis. Environ Health Perspect 2022;130(4):46001. 35475652.ArticlePubMedPMC
- 46. Grove JI, Lo PC, Shrine N, Barwell J, Wain LV, Tobin MD, et al. Identification and characterisation of a rare MTTP variant underlying hereditary non-alcoholic fatty liver disease. JHEP Rep 2023;5(8):100764. 37484212.PubMedPMC
- 47. Potoupni V, Georgiadou M, Chatzigriva E, Polychronidou G, Markou E, Zapantis Gakis C, et al. Circulating tumor necrosis factor-α levels in non-alcoholic fatty liver disease: a systematic review and a meta-analysis. J Gastroenterol Hepatol 2021;36(11):3002–3014. 34289181.ArticlePubMedPDF
- 48. Hsieh CJ, Wang PW, Hu TH. Association of adiponectin gene polymorphism with nonalcoholic fatty liver disease in Taiwanese patients with type 2 diabetes. PLoS One 2015;10(6):e0127521. 26042596.ArticlePubMedPMC
- 49. Roth K, Imran Z, Liu W, Petriello MC. Diet as an exposure source and mediator of per- and polyfluoroalkyl substance (PFAS) toxicity. Front Toxicol 2020;2:601149. 35296120.ArticlePubMedPMC
REFERENCES
REFERENCES
Figure & Data
REFERENCES
Citations

- Effects of mixed exposure to PFAS on adolescent non-alcoholic fatty liver disease: Integrating evidence from human cohorts, toxicogenomics, and animal models to uncover mechanisms and potential target sites
Xiushuai Du, Dan-Lin Li, Xueming Xu, Yitian Wu, Zhiyuan Du, Gang Liang, Yue-Zu Li, Ya-Jie Zheng, Yu Qin, Kelei Qian, Jing Xu, Liping Gao, Gonghua Tao, Chen-Wei Pan, Weiwei Zheng
Journal of Hazardous Materials.2025; 485: 136854. CrossRef - Per- and polyfluoroalkyl substances exposures are associated with non-alcoholic fatty liver disease, particularly fibrosis
Se-Hyun Hwang, Yun-Hee Choi, Da-An Huh, Lita Kim, Kangyeon Park, Jiyoun Lee, Hyeon Jeong Choi, Woohyun Lim, Kyong Whan Moon
Environmental Pollution.2025; 372: 126085. CrossRef - PFAS compounds PFOA and Gen X are teratogenic to sea urchin embryos
Alexandra T. Lion, Sophie M. Bodine, Kelley R. McCutcheon, Mayank Ghogale, Santhan Chandragiri, Deema Abayawardena, Bikram D. Shrestha, Abigail Descoteaux, Kathryn Alvarez, J'nesse A. Balkman, Breelyn Cocke, Athula H. Wikramanayake, Jennifer Schlezinger,
Developmental Biology.2025; 525: 139. CrossRef - Sex Differences in the Association Between the Korean Healthy Eating Index and Liver Enzymes Among Korean Adults
Seong-Uk Baek, Jin-Ha Yoon
Nutrients.2025; 17(14): 2372. CrossRef - Ubiquitous Environmental Exposures and Risk of Hepatocellular Carcinoma: A Narrative Review
Hiwot Mulugeta Abate, Daniel Bujnowski, Ashley Jowell, Cynthia A. Moylan, Cathrine Hoyo, Kara Wegermann
Digestive Diseases and Sciences.2025;[Epub] CrossRef - Association between exposure to VOCs mixture and impaired renal function in Korean adults
Seong-Uk Baek, Jin-Ha Yoon
American Journal of Epidemiology.2025; 194(12): 3520. CrossRef - Diabetes and male fertility disorders
Andrea Graziani, Raffaele Scafa, Giuseppe Grande, Alberto Ferlin
Molecular Aspects of Medicine.2024; 99: 101303. CrossRef
- Figure
- Related articles
-
- Association between outdoor clothing use and serum perfluoroalkyl substances (PFAS): Korean National Environmental Health Survey cycle 4
- Association between sudden work recall and psychological health issues: a cross-sectional analysis of the 6th Korean Working Conditions Survey
- Relationship between the use of hair products and urine benzophenone-3: the Korean National Environmental Health Survey (KoNEHS) cycle 4
- Relationship between crustacean consumption and serum perfluoroalkyl substances (PFAS): the Korean National Environmental Health Survey (KoNEHS) cycle 4
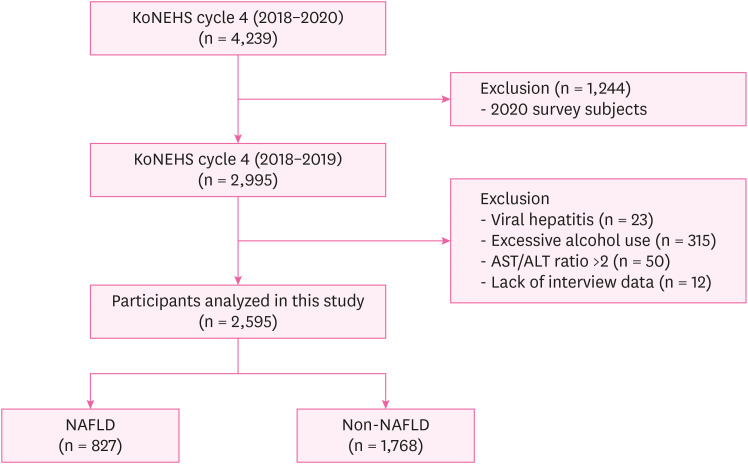
Fig. 1
| Variables | Total (n = 2,595) | Non-NAFLD (n = 1,768) | NAFLD (n = 827) | ||
|---|---|---|---|---|---|
| Age group (years) | 0.232 | ||||
| 19–39 | 547 (35.0) | 368 (34.7) | 179 (35.6) | ||
| 39–59 | 1,054 (37.3) | 723 (37.5) | 331 (37.0) | ||
| ≥ 60 | 994 (27.7) | 677 (27.8) | 317 (27.4) | ||
| Sex | < 0.001 | ||||
| Male | 1,017 (46.0) | 551 (36.6) | 466 (64.1) | ||
| Female | 1,578 (54.0) | 1,217 (63.4) | 361 (35.9) | ||
| BMI (kg/m2) | < 0.001 | ||||
| Normal | 1,383 (54.3) | 1,288 (75.3) | 95 (13.6) | ||
| Obeseb | 1,212 (45.7) | 480 (24.7) | 732 (86.4) | ||
| Drinking status | 0.210 | ||||
| No | 617 (22.0) | 431 (23.4) | 186 (19.4) | ||
| Formerc | 279 (10.1) | 185 (9.4) | 94 (11.5) | ||
| Current | 1,699 (65.5) | 1,152 (67.3) | 547 (69.1) | ||
| Smoking history | < 0.001 | ||||
| No | 1,791 (68.1) | 1,313 (74.5) | 478 (55.6) | ||
| Former | 482 (17.3) | 282 (15.4) | 200 (20.9) | ||
| Current | 322 (14.6) | 173 (10.1) | 149 (23.5) | ||
| Physical activity | 0.473 | ||||
| No | 1,362 (52.0) | 910 (52.3) | 452 (51.4) | ||
| Intermediated | 179 (6.5) | 114 (5.9) | 65 (7.6) | ||
| Vigorous | 1,054 (41.6) | 744 (41.9) | 310 (41.0) | ||
| Monthly household incomee | 0.517 | ||||
| < 2 | 762 (23.0) | 514 (23.0) | 248 (23.0) | ||
| 2–3 | 415 (15.5) | 268 (15.0) | 147 (16.4) | ||
| 3–5 | 695 (28.9) | 474 (28.1) | 221 (30.5) | ||
| ≥ 5 | 723 (32.6) | 512 (33.9) | 211 (30.1) | ||
| Education | 0.299 | ||||
| Less than high school | 731 (20.5) | 401 (21.0) | 230 (19.3) | ||
| High school graduate | 734 (25.5) | 493 (24.3) | 241 (27.9) | ||
| University or higher | 1,130 (54.0) | 774 (54.7) | 356 (52.7) | ||
| Liver enzyme | |||||
| AST (U/L) | 25.30 ± 0.29 | 23.53 ± 0.20 | 28.73 ± 0.67 | < 0.001 | |
| ALT (U/L) | 25.55 ± 0.55 | 19.10 ± 0.26 | 38.05 ± 1.24 | < 0.001 | |
| GGT (U/L) | 27.31 ± 0.83 | 20.38 ± 0.64 | 40.74 ± 1.88 | < 0.001 | |
| Comorbidities | |||||
| Hypertension | 663 (20.8) | 363 (16.8) | 300 (28.6) | < 0.001 | |
| Diabetes | 340 (11.7) | 128 (5.8) | 212 (23.2) | < 0.001 | |
| Dyslipidemia | 779 (28.1) | 430 (21.8) | 349 (40.3) | < 0.001 | |
| Serum PFAS concentration | |||||
| Total PFAS | 30.15 ± 1.03 | 28.76 ± 1.04 | 31.80 ± 1.05 | 0.005 | |
| PFOA | 6.43 ± 1.02 | 6.25 ± 1.03 | 6.44 ± 1.03 | 0.432 | |
| PFOS | 15.07 ± 1.04 | 14.35 ± 1.04 | 16.20 ± 1.06 | 0.003 | |
| PFHxS | 4.17 ± 1.03 | 3.93 ± 1.05 | 4.35 ± 1.05 | 0.078 | |
| PFNA | 2.06 ± 1.02 | 1.96 ± 1.03 | 2.15 ± 1.04 | 0.028 | |
| PFDeA | 0.91 ± 1.02 | 0.88 ± 1.03 | 0.91 ± 1.04 | 0.358 | |
| Variables | Total PFAS | PFOA | PFOS | PFHxS | PFNA | PFDeA | |
|---|---|---|---|---|---|---|---|
| Total (n = 2,595) | 29.29 (9.62–15.32) | 6.31 (2.09–3.06) | 14.74 (5.25–7.89) | 3.96 (1.52–2.61) | 2.04 (0.77–1.15) | 0.86 (0.27–0.44) | |
| Non-NAFLD (n = 1,768) | 28.31 (9.09–15.36) | 6.18 (1.93–2.97) | 14.36 (5.00–7.66) | 3.79 (1.52–2.75) | 1.95 (0.69–1.12) | 0.86 (0.25–0.43) | |
| NAFLD (n = 827) | 30.74 (9.88–16.52) | 6.55 (2.42–3.03) | 15.62 (5.87–9.1) | 4.28 (1.56–2.46) | 2.16 (0.85–1.22) | 0.86 (0.28–0.44) | |
| Sex | |||||||
| Male (n = 1,017) | 30.92 (9.91–14.14) | 6.61 (2.3–2.86) | 15.09 (5.06–8.33) | 4.41 (1.54–2.87) | 2.12 (0.73–1.13) | 0.88 (0.29–0.44) | |
| Female (n = 1,578) | 27.28 (8.86–16.89) | 6.01 (1.91–3.2) | 14.36 (5.4–7.97) | 3.49 (1.34–2.54) | 1.92 (0.7–1.19) | 0.84 (0.24–0.43) | |
| Age group (years) | |||||||
| 19–39 (n = 547) | 19.18 (4.53–6.21) | 4.27 (1.16–1.44) | 9.43 (2.48–3.76) | 2.69 (0.79–1.45) | 1.24 (0.33–0.43) | 0.59 (0.16–0.18) | |
| 40–59 (n = 1,054) | 30.93 (8.09–12.05) | 6.85 (2.12–2.81) | 15.2 (4.12–5.99) | 4.33 (1.48–2.3) | 2.25 (0.74–0.97) | 0.95 (0.26–0.38) | |
| ≥ 60 (n = 994) | 46.53 (11.05–17.15) | 9.21 (2.53–3.9) | 24.44 (6.72–9.18) | 5.79 (2.07–2.72) | 3.36 (1.02–1.26) | 1.31 (0.35–0.56) | |
| BMIa | |||||||
| Normal (n = 1,383) | 27.73 (8.93–14.97) | 5.93 (1.76–2.88) | 14.12 (5.17–7.56) | 3.59 (1.37–2.77) | 1.89 (0.66–1.05) | 0.84 (0.25–0.39) | |
| Obese (n = 1,212) | 31.44 (10.58–15.95) | 6.75 (2.5–3.32) | 15.89 (5.84–8.09) | 4.32 (1.6–2.5) | 2.23 (0.88–1.26) | 0.88 (0.29–0.52) | |
| Serum PFAS | Crudea | Model 1b | Model 2c |
|---|---|---|---|
| Total PFAS | 1.956 (1.234–3.100) | 2.027 (1.106–3.717) | 1.881 (0.993–3.563) |
| PFOA | 1.225 (0.724–2.070) | 1.221 (0.573–2.158) | 1.272 (0.736–2.199) |
| PFOS | 1.917 (1.283–2.863) | 2.094 (1.285–3.414) | 1.981 (1.170–3.356) |
| PFHxS | 1.458 (0.914–2.326) | 1.157 (0.702–1.906) | 1.153 (0.759–1.752) |
| PFNA | 1.658 (1.068–2.574) | 1.741 (0.947–3.200) | 1.755 (0.994–3.098) |
| PFDeA | 1.252 (0.775–2.021) | 1.188 (0.643–2.196) | 1.243 (0.681–2.266) |
| Serum PFAS | Crudea | Model 1b | Model 2c | |||
|---|---|---|---|---|---|---|
| Normald | Obesee | Normal | Obese | Normal | Obese | |
| Total PFAS | 6.401 (1.883–21.758) | 0.680 (0.391–1.183) | 7.642 (1.850–31.560) | 0.897 (0.405–1.988) | 5.207 (1.480–18.323) | 0.916 (0.408–2.054) |
| PFOA | 1.473 (0.431–5.038) | 0.628 (0.360–1.097) | 1.139 (0.235–5.514) | 0.816 (0.400–1.665) | 1.141 (0.256–5.099) | 0.966 (0.466–2.002) |
| PFOS | 7.018 (2.688–18.319) | 0.684 (0.422–1.108) | 9.210 (3.346–25.353) | 0.872 (0.461–1.650) | 7.030 (2.687–18.392) | 0.877 (0.459–1.676) |
| PFHxS | 1.711 (0.867–3.375) | 0.924 (0.539–1.583) | 1.044 (0.417–2.619) | 0.963 (0.550–1.684) | 0.895 (0.331–2.415) | 1.030 (0.594–1.786) |
| PFNA | 3.542 (0.946–13.270) | 0.722 (0.436–1.195) | 4.296 (0.719–25.679) | 1.020 (0.506–2.059) | 2.687 (0.556–12.988) | 1.212 (0.607–2.419) |
| PFDeA | 4.008 (1.022–15.720) | 0.515 (0.315–0.843) | 5.510 (0.994–30.545) | 0.610 (0.286–1.302) | 3.328 (0.907–12.211) | 0.711 (0.320–1.581) |
Values are presented as mean ± standard deviation or sample size (weighted %).
NAFLD: non-alcoholic fatty liver disease; n: unweighted sample size; BMI: body mass index; AST: aspartate aminotransferase; ALT: alanine aminotransferase; GGT: gamma-glutamyl transferase; PFAS: perfluoroalkyl substances; PFOA: perfluorooctanoic acid; PFOS: perfluorooctansulfonate; PFHxS: perfluorohexanesulfonic acid; PFNA: perfluorononanoic acid; PFDeA: perfluorodecanoic acid.
aThe
Values are presented as median (interquartile range).
Quartile of serum PFAS levels were calculated using weighted samples.
PFAS: perfluoroalkyl substances; NAFLD: non-alcoholic fatty liver disease; BMI: body mass index; PFOA: perfluorooctanoic acid; PFOS: perfluorooctansulfonate; PFHxS: perfluorohexanesulfonic acid; PFNA: perfluorononanoic acid; PFDeA: perfluorodecanoic acid; n: unweighted sample size.
aNormal, BMI < 25; obese, BMI ≥ 25.
Values are presented as odds ratio (95% confidence interval).
NAFLD: non-alcoholic fatty liver disease; PFAS: perfluoroalkyl substances; PFOA: perfluorooctanoic acid; PFOS: perfluorooctansulfonate; PFHxS: perfluorohexanesulfonic acid; PFNA: perfluorononanoic acid; PFDeA: perfluorodecanoic acid.
aSerum PFAS, NAFLD based on hepatic steatosis index score; bCrude + adiusted for age(group), sex; cModel 1 + adiusted for smoking history, physical activity, monthly household income, education, hypertension, diabetes, dyslipidemia.
Values are presented as odds ratio (95% confidence interval).
NAFLD: non-alcoholic fatty liver disease; PFAS: perfluoroalkyl substances; BMI: body mass index; PFOA: perfluorooctanoic acid; PFOS: perfluorooctansulfonate; PFHxS: perfluorohexanesulfonic acid; PFNA: perfluorononanoic acid; PFDeA: perfluorodecanoic acid.
aSerum PFAS, NAFLD based on hepatic steatosis index score; bCrude + adiusted for age (group), sex; cModel 1 + adiusted for smoking history, physical activity, monthly household income, education, hypertension, diabetes, dyslipidemia; dNormal, BMI < 25; eObese, BMI ≥ 25.
 KSOEM
KSOEM

 Cite
Cite