Articles
- Page Path
- HOME > Ann Occup Environ Med > Volume 35; 2023 > Article
- Original Article The association of perfluoroalkyl substances (PFAS) exposure and kidney function in Korean adolescents using data from Korean National Environmental Health Survey (KoNEHS) cycle 4 (2018–2020): a cross-sectional study
-
Jisuk Yun
 , Eun-Chul Jang
, Eun-Chul Jang , Soon-Chan Kwon
, Soon-Chan Kwon , Young-Sun Min
, Young-Sun Min , Yong-Jin Lee
, Yong-Jin Lee
-
Annals of Occupational and Environmental Medicine 2023;35:e5.
DOI: https://doi.org/10.35371/aoem.2023.35.e5
Published online: March 15, 2023
Department of Occupational and Environmental Medicine, Soonchunhyang University Cheonan Hospital, Cheonan, Korea.
- Correspondence: Yong-Jin Lee. Department of Occupational and Environmental Medicine, Soonchunhyang University Cheonan Hospital, 31 Suncheonhyang 6-gil, Dongnam-gu, Cheonan 31151, Korea. regina94@schmc.ac.kr
Copyright © 2023 Korean Society of Occupational & Environmental Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background Perfluoroalkyl substances (PFAS) are chemicals widely used in various products in everyday life. Due to its unique strong binding force, the half-life of PFAS is very long, so bioaccumulation and toxicity to the human body are long-standing concerns. In particular, effects on kidney function have recently emerged and there are no studies on the effect of PFAS on kidney function through epidemiological investigations in Korea. From 2018 to 2020, the Korean National Environmental Health Survey (KoNEHS) cycle 4, conducted an epidemiological investigation on the blood concentration of PFAS for the first time in Korea. Based on this data, the relationship between PFAS blood concentration and kidney function was analyzed for adolescents.
-
Methods We investigated 5 types of PFAS and their total blood concentration in 811 middle and high school students, living in Korea and included in KoNEHS cycle 4, and tried to find changes in kidney function in relation to PFAS concentration. After dividing the concentration of each of the 5 PFAS and the total concentration into quartiles, multivariable linear regression was performed to assess the correlation with kidney function. The bedside Schwartz equation was used as an indicator of kidney function.
-
Results As a result of multivariable linear regression, when observing a change in kidney function according to the increase in the concentration of each of the 5 PFAS and their total, a significant decrease in kidney function was confirmed in some or all quartiles.
-
Conclusions In this cross-sectional study of Korean adolescents based on KoNEHS data, a negative correlation between serum PFAS concentration and kidney function was found. A well-designed longitudinal study and continuous follow-up are necessary.
BACKGROUND
METHODS
RESULTS
General characteristics and mean eGFR of the study population
Mean eGFR according to quartile of PFAS
Multivariable regression of PFAS with outcome measure of kidney function
Mean change in eGFR (mL/min/1.73 m2) per 1 ln-serum PFAS using multivariable regression
Mean change in eGFR (mL/min/1.73 m2) per 1 ln-serum PFAS using multivariable regression according to confounders
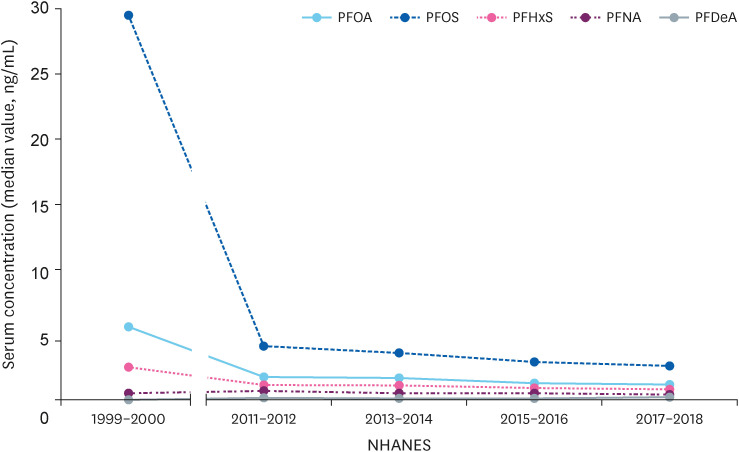
Changes in serum perfluoroalkyl substances concentration of adolescents in 1999–2000 and in the last 4 years in the USA.
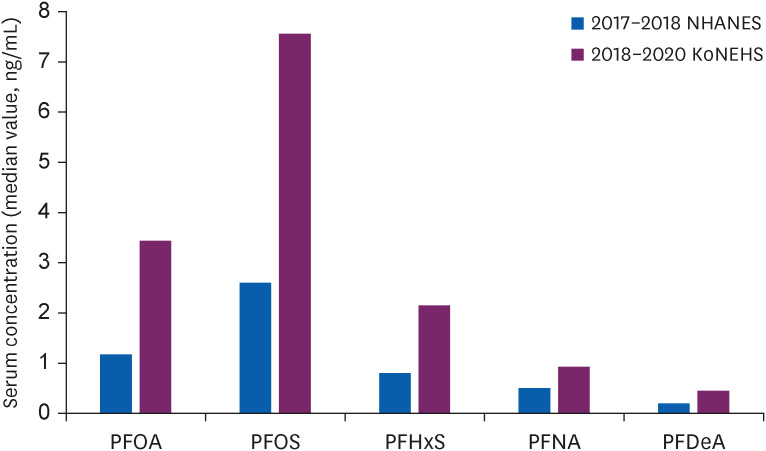
Comparison of recent study results in Korean and USA adolescents.
DISCUSSION
CONCLUSIONS
Acknowledgements
-
Funding: This work was supported by the Soonchunhyang University Research Fund.
-
Competing interests: The authors declare that they have no competing interests.
-
Authors contributions:
Abbreviations
BMI
CI
eGFR
HbA1c
KoNEHS
LOD
NHANES
PFAS
PFDeA
PFHxS
PFNA
PFOA
PFOS
ROS
- 1. OECD. Toward a new comprehensive global database of per-and polyfluoroalkyl substances (PFASs): Summary report on updating the OECD 2007 list of per-and polyfluoroalkyl substances (PFASs). Ser Risk Manag 2018;39:1–24.
- 2. Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 2019;29(2):131–147. 30470793.ArticlePubMedPMCPDF
- 3. Armitage J, Cousins IT, Buck RC, Prevedouros K, Russell MH, MacLeod M, et al. Modeling global-scale fate and transport of perfluorooctanoate emitted from direct sources. Environ Sci Technol 2006;40(22):6969–6975. 17154003.ArticlePubMed
- 4. Yeo MK, Hwang EH, Jeong GH. Distribution characteristics of perfluorinated compounds in major river water and sediment. Anal Sci Technol 2012;25(5):313–323.Article
- 5. National Institute of Environmental Research (KR). A Study on Management of Unregulated Trace Hazardous Compounds in Drinking Water. Incheon, Korea: National Institute of Environmental Research; 2020.
- 6. Son B, Lee L, Yang M, Park S, Pyo H, Lee W, et al. Distribution and risk assessment of perfluorinated compounds (PFCs) in major drinking water treatment plants, Korea. J Korean Soc Water Wastewater 2017;31(6):491–499.Article
- 7. Kirk M, Smurthwaite K, Braunig J, Trevenar S, D’Este C, Lucas R, et al. The PFAS Health Study: Systematic Literature Review. Canberra, Australia: The Australian National University; 2018.
- 8. Ferrari F, Orlando A, Ricci Z, Ronco C. Persistent pollutants: focus on perfluorinated compounds and kidney. Curr Opin Crit Care 2019;25(6):539–549. 31524719.ArticlePubMed
- 9. Stanifer JW, Stapleton HM, Souma T, Wittmer A, Zhao X, Boulware LE. Perfluorinated chemicals as emerging environmental threats to kidney health: a scoping review. Clin J Am Soc Nephrol 2018;13(10):1479–1492. 30213782.PubMedPMC
- 10. Cui L, Zhou QF, Liao CY, Fu JJ, Jiang GB. Studies on the toxicological effects of PFOA and PFOS on rats using histological observation and chemical analysis. Arch Environ Contam Toxicol 2009;56(2):338–349. 18661093.ArticlePubMedPDF
- 11. Qian Y, Ducatman A, Ward R, Leonard S, Bukowski V, Lan Guo N, et al. Perfluorooctane sulfonate (PFOS) induces reactive oxygen species (ROS) production in human microvascular endothelial cells: role in endothelial permeability. J Toxicol Environ Health A 2010;73(12):819–836. 20391123.ArticlePubMedPMC
- 12. Zhang B, He Y, Huang Y, Hong D, Yao Y, Wang L, et al. Novel and legacy poly- and perfluoroalkyl substances (PFASs) in indoor dust from urban, industrial, and e-waste dismantling areas: the emergence of PFAS alternatives in China. Environ Pollut 2020;263(Pt A):114461. 32251969.ArticlePubMed
- 13. Jain RB. Perfluoroalkyl acids and their isomers, diabetes, anemia, and albuminuria: variabilities with deteriorating kidney function. Ecotoxicol Environ Saf 2021;208:111625. 33396145.ArticlePubMed
- 14. Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and comparisons with NHANES 1999-2000. Environ Health Perspect 2007;115(11):1596–1602. 18007991.ArticlePubMedPMC
- 15. PFAS in the U.S. population. Updated 2022]. Accessed November 14, 2022]. https://www.atsdr.cdc.gov/pfas/health-effects/us-population.html .
- 16. Landrigan PJ, Goldman LR. Children’s vulnerability to toxic chemicals: a challenge and opportunity to strengthen health and environmental policy. Health Aff (Millwood) 2011;30(5):842–850. 21543423.ArticlePubMed
- 17. Renal function tests. Updated 2022]. Accessed November 14, 2022]. https://www.ncbi.nlm.nih.gov/books/NBK507821 .
- 18. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 2021;99(3S):S1–87. 33637192.PubMed
- 19. Staples A, LeBlond R, Watkins S, Wong C, Brandt J. Validation of the revised Schwartz estimating equation in a predominantly non-CKD population. Pediatr Nephrol 2010;25(11):2321–2326. 20652327.ArticlePubMedPDF
- 20. Comptox chemicals dashboard. Updated 2022]. Accessed November 14, 2022]. https://comptox.epa.gov/dashboard/chemical-lists/PFASSTRUCTV5 .
- 21. Kazancioğlu R. Risk factors for chronic kidney disease: an update. Kidney Int Suppl (2011) 2013;3(4):368–371. 25019021.ArticlePubMedPMC
- 22. Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017;140(3):e20171904. 28827377.PubMed
- 23. Hur KY, Moon MK, Park JS, Kim SK, Lee SH, Yun JS, et al. 2021 clinical practice guidelines for diabetes mellitus of the Korean Diabetes Association. Diabetes Metab J 2021;45(4):461–481. 34352984.PubMedPMC
- 24. García-Esquinas E, Loeffler LF, Weaver VM, Fadrowski JJ, Navas-Acien A. Kidney function and tobacco smoke exposure in US adolescents. Pediatrics 2013;131(5):e1415–e1423. 23569089.ArticlePubMedPMCPDF
- 25. Lee W, Lee S, Roh J, Won JU, Yoon JH. The association between involuntary smoking exposure with urine cotinine level and blood cadmium level in general non-smoking populations. J Korean Med Sci 2017;32(4):568–575. 28244280.ArticlePubMedPMCPDF
- 26. Tonelli M, Isles C, Craven T, Tonkin A, Pfeffer MA, Shepherd J, et al. Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation 2005;112(2):171–178. 15998677.ArticlePubMed
- 27. Lim JS, Kim EY, Kim JH, Yoo JH, Yi KH, Chae HW, et al. 2017 clinical practice guidelines for dyslipidemia of Korean children and adolescents. Ann Pediatr Endocrinol Metab 2020;25(4):199–207. 33401878.ArticlePubMedPMCPDF
- 28. Korean National Institute of Environmental Research. Guidelines for Using Raw Materials for Korean National Environmental Health Survey (Child, Adolescent)-the Fourth Stage (2018–2020). Incheon, Korea: Korean National Institute of Environmental Research; 2022.
- 29. Shankar A, Xiao J, Ducatman A. Perfluoroalkyl chemicals and chronic kidney disease in US adults. Am J Epidemiol 2011;174(8):893–900. 21873601.ArticlePubMedPMC
- 30. Watkins DJ, Josson J, Elston B, Bartell SM, Shin HM, Vieira VM, et al. Exposure to perfluoroalkyl acids and markers of kidney function among children and adolescents living near a chemical plant. Environ Health Perspect 2013;121(5):625–630. 23482063.ArticlePubMedPMC
- 31. Kataria A, Trachtman H, Malaga-Dieguez L, Trasande L. Association between perfluoroalkyl acids and kidney function in a cross-sectional study of adolescents. Environ Health 2015;14(1):89. 26590127.ArticlePubMedPMCPDF
- 32. Poothong S, Papadopoulou E, Padilla-Sánchez JA, Thomsen C, Haug LS. Multiple pathways of human exposure to poly- and perfluoroalkyl substances (PFASs): from external exposure to human blood. Environ Int 2020;134:105244. 31711019.ArticlePubMed
- 33. Fàbrega F, Kumar V, Schuhmacher M, Domingo JL, Nadal M. PBPK modeling for PFOS and PFOA: validation with human experimental data. Toxicol Lett 2014;230(2):244–251. 24440341.ArticlePubMed
- 34. Pérez F, Nadal M, Navarro-Ortega A, Fàbrega F, Domingo JL, Barceló D, et al. Accumulation of perfluoroalkyl substances in human tissues. Environ Int 2013;59:354–362. 23892228.ArticlePubMed
- 35. Han X, Nabb DL, Russell MH, Kennedy GL, Rickard RW. Renal elimination of perfluorocarboxylates (PFCAs). Chem Res Toxicol 2012;25(1):35–46. 21985250.ArticlePubMed
- 36. Zhang Y, Beesoon S, Zhu L, Martin JW. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ Sci Technol 2013;47(18):10619–10627. 23980546.ArticlePubMed
- 37. Worley RR, Yang X, Fisher J. Physiologically based pharmacokinetic modeling of human exposure to perfluorooctanoic acid suggests historical non drinking-water exposures are important for predicting current serum concentrations. Toxicol Appl Pharmacol 2017;330:9–21. 28684146.ArticlePubMedPMC
- 38. Yang CH, Glover KP, Han X. Characterization of cellular uptake of perfluorooctanoate via organic anion-transporting polypeptide 1A2, organic anion transporter 4, and urate transporter 1 for their potential roles in mediating human renal reabsorption of perfluorocarboxylates. Toxicol Sci 2010;117(2):294–302. 20639259.ArticlePubMed
- 39. Moon J. Perfluoroalkyl substances (PFASs) exposure and kidney damage: Causal interpretation using the US 2003-2018 National Health and Nutrition Examination Survey (NHANES) datasets. Environ Pollut 2021;288:117707. 34252714.ArticlePubMed
- 40. Blake BE, Pinney SM, Hines EP, Fenton SE, Ferguson KK. Associations between longitudinal serum perfluoroalkyl substance (PFAS) levels and measures of thyroid hormone, kidney function, and body mass index in the Fernald Community Cohort. Environ Pollut 2018;242(Pt A):894–904. 30373035.ArticlePubMedPMC
- 41. Lin PD, Cardenas A, Hauser R, Gold DR, Kleinman KP, Hivert MF, et al. Per- and polyfluoroalkyl substances and kidney function: follow-up results from the Diabetes Prevention Program trial. Environ Int 2021;148:106375. 33482440.ArticlePubMedPMC
- 42. Weinstein JR, Anderson S. The aging kidney: physiological changes. Adv Chronic Kidney Dis 2010;17(4):302–307. 20610357.ArticlePubMedPMC
- 43. De Silva AO, Armitage JM, Bruton TA, Dassuncao C, Heiger-Bernays W, Hu XC, et al. PFAS exposure pathways for humans and wildlife: a synthesis of current knowledge and key gaps in understanding. Environ Toxicol Chem 2021;40(3):631–657. 33201517.ArticlePubMedPMCPDF
- 44. Rahman MF, Peldszus S, Anderson WB. Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: a review. Water Res 2014;50:318–340. 24216232.ArticlePubMed
- 45. Detected ‘perfluorinated compounds’ in frying pan coating materials in half of domestic cosmetics survey subjects. Updated 2021]. Accessed November 14, 2022]. http://kfem.or.kr/?p=220037 .
- 46. Calderon-Margalit R, Golan E, Twig G, Leiba A, Tzur D, Afek A, et al. History of childhood kidney disease and risk of adult end-stage renal disease. N Engl J Med 2018;378(5):428–438. 29385364.ArticlePubMed
- 47. Pottel H, Dubourg L, Goffin K, Delanaye P. Alternatives for the bedside Schwartz equation to estimate glomerular filtration rate in children. Adv Chronic Kidney Dis 2018;25(1):57–66. 29499888.ArticlePubMed
References
Figure & Data
REFERENCES
Citations

- Perfluorooctanoic acid induced lung toxicity via TGF-β1/Smad pathway, crosstalk between airway hyperresponsiveness and fibrosis: withdrawal impact
Arwa A. Elsheikh, Amany Mohamed Shalaby, Mohamed Ali Alabiad, Noha Ali Abd-Almotaleb, Eman El-Sayed Khayal
Environmental Science and Pollution Research.2025; 32(9): 4989. CrossRef - Environmental disease monitoring by regional Environmental Health
Centers in Korea: a narrative review
Myung-Sook Park, Hwan-Cheol Kim, Woo Jin Kim, Yun-Chul Hong, Won-Jun Choi, Seock-Yeon Hwang, Jiho Lee, Young-Seoub Hong, Yong-Dae Kim, Seong-Chul Hong, Joo Hyun Sung, Inchul Jeong, Kwan Lee, Won-Ju Park, Hyun-Joo Bae, Seong-Yong Yoon, Cheolmin Lee, Kyoung
The Ewha Medical Journal.2025;[Epub] CrossRef - Association Between Perfluoroalkyl Substance (PFAS) Exposure and Nonalcoholic Fatty Liver Disease in Korean Adults: Results From the KoNEHS 2018–2020: A Cross‐Sectional Study
Jisuk Yun, Young‐Sun Min
American Journal of Industrial Medicine.2025;[Epub] CrossRef - Association of Combined Per- and Polyfluoroalkyl Substances and Metals with Chronic Kidney Disease
Issah Haruna, Emmanuel Obeng-Gyasi
International Journal of Environmental Research and Public Health.2024; 21(4): 468. CrossRef - Leveraging Machine Learning for a Comprehensive Assessment of PFAS Nephrotoxicity
Anirudh Mazumder, Kapil Panda
Advances in Science, Technology and Engineering Systems Journal.2024; 9(3): 62. CrossRef - Clinical, histological, molecular, and toxicokinetic renal outcomes of per-/polyfluoroalkyl substances (PFAS) exposure: Systematic review and meta-analysis
Jidapa Hanvoravongchai, Methasit Laochindawat, Yusuke Kimura, Nathan Mise, Sahoko Ichihara
Chemosphere.2024; 368: 143745. CrossRef
- Figure
- Related articles
-
- Relationship between the use of hair products and urine benzophenone-3: the Korean National Environmental Health Survey (KoNEHS) cycle 4
- Relationship between crustacean consumption and serum perfluoroalkyl substances (PFAS): the Korean National Environmental Health Survey (KoNEHS) cycle 4
- Association between serum perfluoroalkyl substances concentrations and non-alcoholic fatty liver disease among Korean adults: a cross-sectional study using the National Environmental Health Survey cycle 4
- Relationship between the use of plastics in refrigerator food storage and urine phthalate metabolites: the Korean National Environmental Health Survey (KoNEHS) cycle 3
- Relationship between shellfish consumption and urinary phthalate metabolites: Korean National Environmental Health Survey (KoNEHS) cycle 3 (2015-2017)
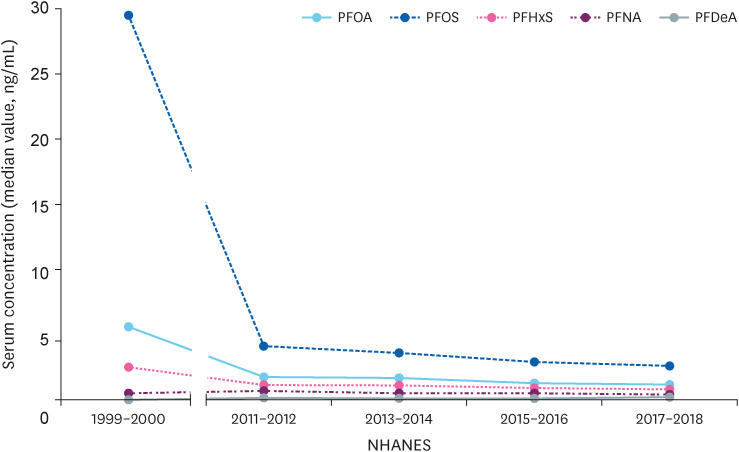
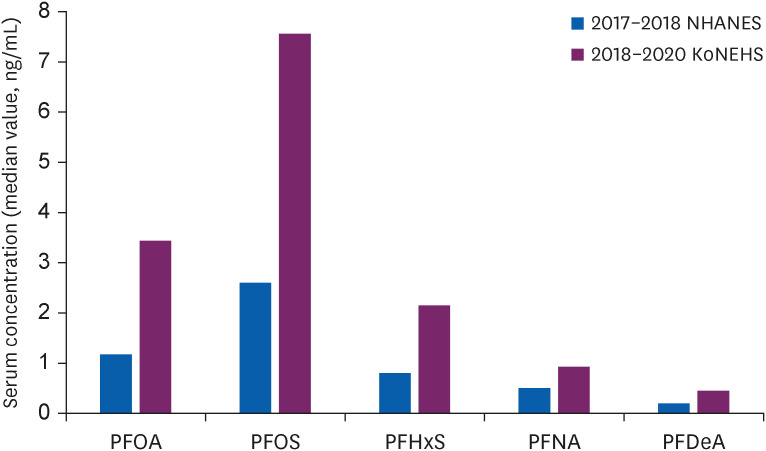
Fig. 1
Fig. 2
| Characteristics | Unweighted sample size (n = 811) | eGFR (mL/min/1.73 m2) | ||
|---|---|---|---|---|
| Sex | 94.67 (93.49–95.85) | < 0.001*** | ||
| Male | 379 (46.7) | 85.35 (83.94–86.76) | ||
| Female | 432 (53.3) | 102.84 (101.38–104.31) | ||
| Age (years) | < 0.001*** | |||
| 12 | 122 (15.0) | 104.82 (102.05–107.59) | ||
| 13 | 113 (13.9) | 99.21 (96.50–101.87) | ||
| 14 | 128 (15.8) | 97.03 (94.16–99.89) | ||
| 15 | 154 (19.0) | 90.74 (88.30–93.19) | ||
| 16 | 156 (19.2) | 89.17 (86.87–91.47) | ||
| 17 | 138 (17.0) | 90.38 (86.90–93.87) | ||
| Household income (million won)b | 0.253 | |||
| < 1 (803 $) | 10 (1.2) | 89.32 (77.47–101.17) | ||
| 1–2 (803–1,606 $) | 42 (5.2) | 97.93 (92.64–103.23) | ||
| 2–3 (1,606–2,409 $) | 100 (12.3) | 95.18 (91.06–99.29) | ||
| 3–5 (2,409–4,014 $) | 269 (33.2) | 95.90 (93.88–97.93) | ||
| 5–7 (4,014–5,620 $) | 196 (24.2) | 92.65 (90.39–94.92) | ||
| ≥ 7 (5,620 $) | 147 (18.1) | 93.59 (90.86–96.31) | ||
| unaware | 47 (5.8) | 96.51 (91.81–101.21) | ||
| BMI (kg/m2) | < 0.001*** | |||
| < 18.5 | 106 (13.1) | 100.82 (97.78–103.85) | ||
| 18.5–25 | 540 (66.6) | 93.98 (92.59–95.38) | ||
| ≥ 25 | 165 (20.3) | 92.96 (89.97–95.94) | ||
| HbA1c (%) | 0.533 | |||
| < 6.5 | 808 (99.6) | 94.74 (93.55–95.87) | ||
| ≥ 6.5 | 3 (0.4) | 88.50 (64.28–109.89) | ||
| Urinary cotinine (ng/mL) | < 0.001*** | |||
| < 1.93 | 270 (33.3) | 98.37 (96.20–100.53) | ||
| 1.94–3.43 | 271 (33.3) | 93.95 (91.88–96.01) | ||
| ≥ 3.44 | 270 (33.3) | 91.69 (89.84–93.5) | ||
| Total cholesterol (mg/dL) | 0.734 | |||
| < 170 | 598 (73.7) | 94.55 (93.17–95.97) | ||
| ≥ 170 | 213 (26.3) | 95.01 (92.69–97.57) | ||
| Abnormal blood pressurec | < 0.001*** | |||
| No | 635 (78.3) | 95.74 (94.54–97.13) | ||
| Yes | 176 (21.7) | 90.82 (88.09–93.58) | ||
| Serum perfluoroalkyl chemical level (μg/L) | Unweighted sample size (n = 811) | eGFR (mL/min/1.73 m2) | ||
|---|---|---|---|---|
| Total PFAS | < 0.001*** | |||
| Quartile 1 (< 11.92 μg/L) | 203 | 99.37 (96.84–101.90) | ||
| Quartile 2 (11.92 to 15.89 μg/L) | 201 | 93.65 (91.30–96.00) | ||
| Quartile 3 (15.89 to 21.94 μg/L) | 205 | 93.11 (91.01–95.22) | ||
| Quartile 4 (> 21.94 μg/L) | 202 | 92.56 (90.16–94.96) | ||
| PFOA | < 0.001*** | |||
| Quartile 1 (< 2.70 μg/L) | 203 | 98.31 (95.64–100.97) | ||
| Quartile 2 (2.70 to 3.44 μg/L) | 203 | 95.50 (93.35–97.66) | ||
| Quartile 3 (3.44 to 4.76 μg/L) | 204 | 93.21 (90.90–95.52) | ||
| Quartile 4 (> 4.76 μg/L) | 201 | 91.69 (89.41–93.96) | ||
| PFOS | 0.022* | |||
| Quartile 1 (< 5.71 μg/L) | 202 | 97.04 (94.71–99.37) | ||
| Quartile 2 (5.71 to 7.61 μg/L) | 204 | 95.96 (93.41–98.52) | ||
| Quartile 3 (7.62 to 11.08 μg/L) | 203 | 92.80 (90.65–94.95) | ||
| Quartile 4 (> 11.08 μg/L) | 202 | 92.88 (90.46–95.29) | ||
| PFHxS | < 0.001*** | |||
| Quartile 1 (< 1.42 μg/L) | 202 | 98.98 (96.45–101.51) | ||
| Quartile 2 (1.42 to 2.16 μg/L) | 204 | 94.64 (92.28–96.99) | ||
| Quartile 3 (2.16 to 3.47 μg/L) | 203 | 92.05 (89.78–94.32) | ||
| Quartile 4 (> 3.47 μg/L) | 202 | 93.04 (90.79–95.28) | ||
| PFNA | < 0.001*** | |||
| Quartile 1 (< 0.70 μg/L) | 203 | 99.63 (96.91–102.35) | ||
| Quartile 2 (0.70 to 0.93 μg/L) | 202 | 93.65 (91.51–95.79) | ||
| Quartile 3 (0.93 to 1.22 μg/L) | 204 | 94.03 (91.97–96.10) | ||
| Quartile 4 (> 1.22 μg/L) | 202 | 91.39 (88.99–93.78) | ||
| PFDeA | 0.003** | |||
| Quartile 1 (< 0.36 μg/L) | 204 | 97.54 (94.93–100.16) | ||
| Quartile 2 (0.36 to 0.45 μg/L) | 201 | 94.48 (92.36–96.60) | ||
| Quartile 3 (0.46 to 0.56 μg/L) | 203 | 95.31 (92.96–97.66) | ||
| Quartile 4 (> 0.56 μg/L) | 203 | 91.36 (89.04–93.69) | ||
| Serum perfluoroalkyl chemical level (μg/L) | Unweighted sample size (n = 811) | Multivariable adjusted change in eGFRa (mL/min/1.73 m2) | |
|---|---|---|---|
| Total PFAS | |||
| Quartile 1 (< 11.92 μg/L) | 203 | Reference | |
| Quartile 2 (11.92 to 15.89 μg/L) | 201 | −4.04 (−7.45, −0.59)** | |
| Quartile 3 (15.89 to 21.94 μg/L) | 205 | −5.13 (−7.98, −2.27)*** | |
| Quartile 4 (> 21.94 μg/L) | 202 | −5.17 (−8.61, −1.73)* | |
| PFOA | |||
| Quartile 1 (< 2.70 μg/L) | 203 | Reference | |
| Quartile 2 (2.70 to 3.44 μg/L) | 203 | −1.57 (−4.04, 0.90) | |
| Quartile 3 (3.44 to 4.76 μg/L) | 204 | −3.96 (−6.68, −1.23)** | |
| Quartile 4 (> 4.76 μg/L) | 201 | −4.16 (−7.44, −0.87)* | |
| PFOS | |||
| Quartile 1 (< 5.71 μg/L) | 202 | Reference | |
| Quartile 2 (5.71 to 7.61 μg/L) | 204 | −1.46 (−4.51, 1.59) | |
| Quartile 3 (7.62 to 11.08 μg/L) | 203 | −3.39 (−5.84, −0.94)** | |
| Quartile 4 (> 11.08 μg/L) | 202 | −3.13 (−5.64, −0.61)* | |
| PFHxS | |||
| Quartile 1 (< 1.42 μg/L) | 202 | Reference | |
| Quartile 2 (1.42 to 2.16 μg/L) | 204 | −3.85 (−6.78, −0.93)* | |
| Quartile 3 (2.16 to 3.47 μg/L) | 203 | −3.63 (−6.61, −0.65)* | |
| Quartile 4 (> 3.47 μg/L) | 202 | −5.24 (−8.59, −1.88)** | |
| PFNA | |||
| Quartile 1 (< 0.70 μg/L) | 203 | Reference | |
| Quartile 2 (0.70 to 0.93 μg/L) | 202 | −3.77 (−6.49, −1.05)** | |
| Quartile 3 (0.93 to 1.22 μg/L) | 204 | −3.32 (−5.80, −0.83)** | |
| Quartile 4 (> 1.22 μg/L) | 202 | −4.20 (−7.35, −1.04)* | |
| PFDeA | |||
| Quartile 1 (< 0.36 μg/L) | 204 | Reference | |
| Quartile 2 (0.36 to 0.45 μg/L) | 201 | −1.45 (−4.25, 1.35) | |
| Quartile 3 (0.46 to 0.56 μg/L) | 203 | −2.28 (−5.09, 0.54) | |
| Quartile 4 (> 0.56 μg/L) | 203 | −3.79 (−6.74, −0.84)* | |
| Serum perfluoroalkyl chemicals | Multivariable adjusted change in eGFR (mL/min/1.73 m2) | |
|---|---|---|
| Model 1a | Model 2b | |
| Total PFAS | −2.81 (−5.50, −0.12)* | −3.31 (−5.98, −0.64)* |
| PFOA | −2.62 (−5.40, 0.17) | −3.09 (−5.80, −0.38)* |
| PFOS | −2.24 (−3.96, −0.53)* | −2.50 (−4.20, −0.80)** |
| PFHxS | −1.20 (−3.06, 0.67) | −1.53 (−3.35, 0.29) |
| PFNA | −2.21 (−4.73, 0.31) | −2.45 (−4.91, 0.02) |
| PFDeA | −2.50 (−5.26, 0.25) | −3.05 (−5.77, −0.32)* |
| Characteristics | Multivariable adjusted change in eGFRa (mL/min/1.73 m2) | ||||||
|---|---|---|---|---|---|---|---|
| Total PFAS | PFOA | PFOS | PFHxS | PFNA | PFDeA | ||
| Sex | |||||||
| Male | −2.27 (−4.84, 0.31) | −2.45 (−5.58, 0.68) | −1.68 (−3.57, 0.20) | −1.50 (−3.76, 0.76) | −2.23 (−5.30, 0.84) | −2.57 (−5.70, 0.57) | |
| Female | −4.50 (−8.79, −0.20)* | −3.75 (−7.85, 0.35) | −3.44 (−6.31, −0.57)* | −1.60 (−3.85, 0.64) | −3.02 (−6.95, 0.92) | −3.72 (−8.37, 0.92) | |
| Age (years) | |||||||
| < 15 | −1.66 (−4.81, 1.49) | 0.38 (−2.78, 3.54) | −1.51 (−3.90, 0.88) | −1.14 (−3.63, 0.35) | 0.89 (−3.19, 4.97) | 0.82 (−3.22, 0.41) | |
| ≥ 15 | −3.79 (−7.84, 0.26) | −6.30 (−10.56, −2.04)** | −2.80 (−5.11, −0.49)* | −1.25 (−4.19, 1.70) | −4.80 (−8.17, −1.43)** | −5.76 (−9.46, −2.06)** | |
| BMI (kg/m2) | |||||||
| < 18.5 | −3.98 (−0.83, 0.32) | −2.20 (−8.18, 3.77) | −5.27 (−8.52, −2.03)** | −0.11 (−2.46, 2.25) | −4.35 (−11.16, 2.47) | −1.75 (−8,42, 4.93) | |
| 18.5–25 | −2.23 (−5.14, 0.68) | −2.19 (−4.80, 0.42) | −2.05 (−4.05, −0.05)* | −0.55 (−2.41, 1.30) | −2.22 (−5.02, 0.57) | −3.14 (−6.46, 0.19) | |
| ≥ 25 | −6.53 (−12.72, −0.34)* | −7.40 (−14.17, −0.62)* | −2.00 (−7.21, 3.21) | −6.26 (−10.00, −2.52)** | −4.63 (−10.77, 1.51) | −4.31 (−10.05, 1.43) | |
| Urinary cotinine (ng/mL) | |||||||
| < 1.93 | −3.33 (−8.50, 1.84) | −3.23 (−8.91, 2.45) | −1.63 (−5.44, 2.17) | −2.85 (−5.51, −0.19)* | −2.84 (−8.02, 2.35) | −4.34 (−10.56, 1.89) | |
| 1.94–3.43 | −3.58 (−7.97, 0.82) | −3.74 (−7.69, 0.20) | −3.06 (−6.29, 0.18) | −0.69 (−3.28, 1.90) | −4.12 (−8.36, 0.13) | −1.05 (−5.26, 3.17) | |
| ≥ 3.44 | −2.37 (−4.79, 0.04) | −1.31 (−4.65, 2.04) | −2.20 (−4.01, −0.38)* | −1.31 (−3.97, 1.34) | −0.67 (−4.02, 2.69) | −2.91 (−6.37, 0.57) | |
| Total cholesterol (mg/dL) | |||||||
| < 170 | −2.30 (−5.00, 0.39) | −3.69 (−6.55, −0.82)* | −1.43 (−3.25, 0.39) | −1.09 (−3.04, 0.86) | −3.55 (−6.31, −0.79)* | −3.66 (−6.69, −0.64)* | |
| ≥ 170 | −5.72 (−11.24, −0.21)* | −1.79 (−8.47, 4.89) | −4.51 (−8.34, −0.68)* | −3.01 (−5.78, −0.23)* | −0.59 (−6.48, 5.31) | −0.93 (−7.68, 5.82) | |
| Abnormal blood pressureb | |||||||
| No | −1.97 (−4.98, 1.04) | −2.08 (−5.06, 0.90) | −1.58 (−3.46, 0.30) | −0.87 (−2.84, 1.09) | −1.38 (−4.19, 1.42) | −1.68 (−4.93, 1.58) | |
| Yes | −6.37 (−11.21, −1.53)* | −5.57 (−11.72, 0.58) | −4.85 (−9.06, −0.65)* | −3.39 (−7.36, 0.59) | −6.42 (−13.20, 0.36) | −9.27 (−16.42, −2.11)* | |
Values are presented as number (%) or mean (95% confidence interval).
BMI: body mass index; eGFR: estimated glomerular filtration rate; HbA1c: hemoglobin A1c.
aThe
bAn exchange rate of 1,245.5 won per dollar was applied.
cAbnormal blood pressure was defined as systolic blood pressure > 140 mmHg, diastolic blood pressure > 90 mmHg, or currently taking anti-hypertensive drugs.
***
Values are presented as number or mean (95% confidence interval).
PFAS: perfluoroalkyl substances; PFOA: perfluorooctanoic acid; PFOS: perfluorooctansulfonate; PFHxS: perfluorohexanesulfonic acid; PFNA: perfluorononanoic acid; PFDeA: perfluorodecanoic acid’ eGFR: estimated glomerular filtration rate.
aThe
*
Values are presented as mean (95% confidence interval).
PFAS: perfluoroalkyl substances; PFOA: perfluorooctanoic acid; PFOS: perfluorooctansulfonate; PFHxS: perfluorohexanesulfonic acid; PFNA: perfluorononanoic acid; PFDeA: perfluorodecanoic acid; eGFR: estimated glomerular filtration rate.
aAdjusted for sex, age, household income, body mass index, urinary cotinine, hemoglobin A1c, total cholesterol, abnormal blood pressure
*
Values are presented as mean (95% confidence interval).
PFAS: perfluoroalkyl substances; PFOA: perfluorooctanoic acid; PFOS: perfluorooctansulfonate; PFHxS: perfluorohexanesulfonic acid; PFNA: perfluorononanoic acid; PFDeA: perfluorodecanoic acid; eGFR: estimated glomerular filtration rate.
aAdjusted for sex and age.
bAdjusted for sex, age, household income, body mass index, urinary cotinine, hemoglobin A1c, total cholesterol and abnormal blood pressure.
*
Values are presented as mean (95% confidence interval).
PFAS: perfluoroalkyl substances; PFOA: perfluorooctanoic acid; PFOS: perfluorooctansulfonate; PFHxS: perfluorohexanesulfonic acid; PFNA: perfluorononanoic acid; PFDeA: perfluorodecanoic acid; eGFR: estimated glomerular filtration rate; BMI: body mass index.
aAdjusted for sex, age, household income, body mass index, urinary cotinine, hemoglobin A1c, total cholesterol, abnormal blood pressure
bAbnormal blood pressure was defined as systolic blood pressure > 140 mmHg, diastolic blood pressure > 90 mmHg, or currently taking anti-hypertensive drugs.
*
 KSOEM
KSOEM

 Cite
Cite

