Articles
- Page Path
- HOME > Ann Occup Environ Med > Volume 32; 2020 > Article
- Research Article The association between mercury concentrations and lipid profiles in the Korean National Environmental Health Survey (KoNEHS) cycle 3
-
Soo Ho Sohn1
 , Han Cheol Heo1
, Han Cheol Heo1 , Seongmin Jo1
, Seongmin Jo1 , Chulyong Park1
, Chulyong Park1 , Joon Sakong2
, Joon Sakong2
-
Annals of Occupational and Environmental Medicine 2020;32:e19.
DOI: https://doi.org/10.35371/aoem.2020.32.e19
Published online: June 22, 2020
1Department of Occupational and Environmental Medicine, Yeungnam University Hospital, Daegu, Korea.
2Department of Preventive Medicine and Public Health, College of Medicine, Yeungnam University, Daegu, Korea.
- Correspondence: Joon Sakong. Department of Preventive Medicine and Public Health, College of Medicine, Yeungnam University, 170 Hyeonchung-ro, Nam-gu, Daegu 42415, Korea. jjsakong@gmail.com
Copyright © 2020 Korean Society of Occupational & Environmental Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background High concentrations of mercury intake from seafood are known to cause various side effects in humans, including on the nervous system. Various studies have reported the effects of mercury concentrations in humans; however, the association between dyslipidemia, a cardiovascular disease risk factor, and mercury remains controversial. Therefore, this study aimed to investigate the association between mercury accumulation and cholesterol concentrations in a Korean population.
-
Methods We analyzed data of a sample of 3,228 respondents obtained from the Korean National Environmental Health Survey cycle 3, surveyed between 2015 and 2017, to determine how lipid profiles changed according to the blood mercury concentrations (BHg) and urine mercury concentrations (UHg). Multiple regression analysis was used to determine the effects of mercury concentrations among various factors affecting blood cholesterol levels.
-
Results The arithmetic mean (AM) of BHg was 2.91 (2.81–3.02) μg/L, and the geometric mean (GM) was 2.71 (2.59–2.85) μg/L. The AM of UHg was 0.52 (0.48–0.56) μg/L, and the GM was 0.35 (0.33–0.38) μg/L. Lipid profiles were more related to the BHg than to the UHg. Total cholesterol (total-C), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) levels increased significantly as BHg increased in males, and total-C, triglyceride, and LDL-C levels increased significantly in females. Multiple regression analysis indicated that BHg were significantly associated with total-C, HDL-C, and LDL-C levels.
-
Conclusions We found an association between mercury exposure and the risk of dyslipidemia; however, further studies are required to elucidate a causal association.
BACKGROUND
METHODS
RESULTS
General characteristics of study population
Blood & urine mercury concentrations of study population by sex, age, BMI, smoking, drinking, education level
Blood lipid levels according to quartile groups of blood and urine mercury levels in males and females
Multiple regression analysis between lipid profiles and cardiovascular risk factors
Lipid profiles according to HBM I, II
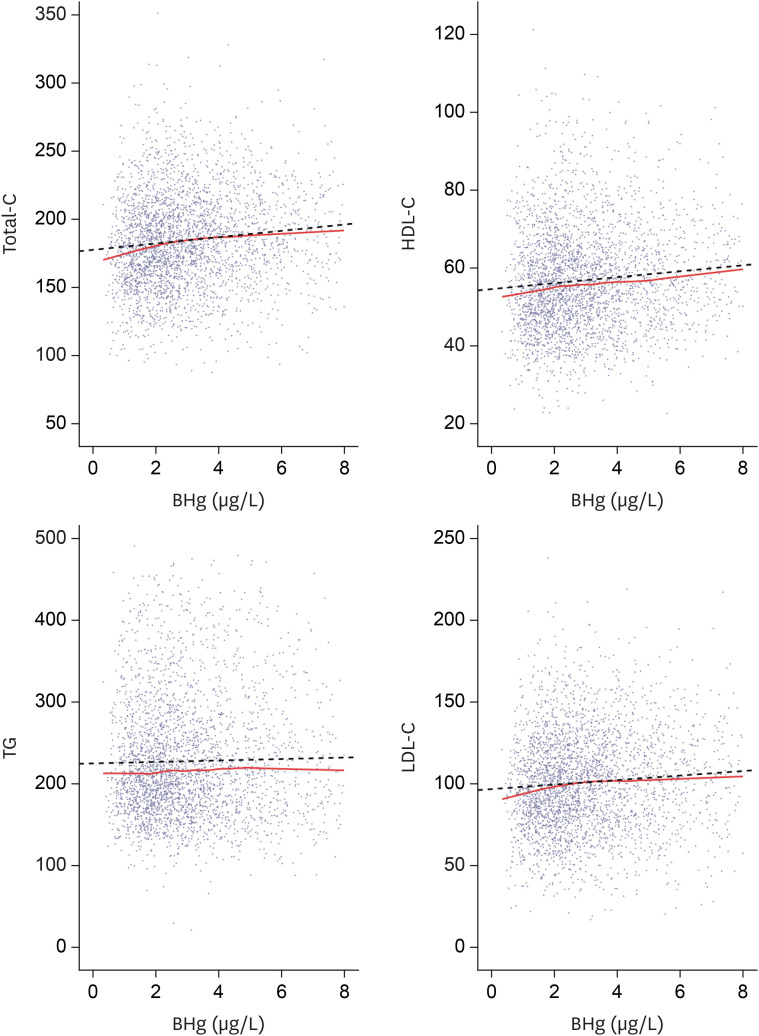
Lipid profiles and blood mercury concentrations by scatter plot. Total-C, HDL-C, LDL-C show a tendency to increase according to blood mercury concentrations. Solid line represents locally estimated scatterplot smoothing. Dotted line represents regression.
Concentrations of blood mercury according to the frequency of fish and shellfish intake
DISCUSSION
CONCLUSIONS
Abbreviations
KoNEHS
Total-C
HDL-C
LDL-C
TG
KNHANES
QC
SD
BMI
LOESS
IRB
HBM
PON1
PUFA
GM
AM
CI
BHg
UHg
SE
-
Funding: This work was supported by the 2019 Yeungnam University Research Grants.
-
Competing interests: The authors declare that they have no competing interests.
-
Availability of data and materials: This study used data from the 3rd KoNEHS (2015–2017), which was conducted by the National Institute of Environmental Research of Korea.
-
Author Contributions:
NOTES
- 1. You CH, Kim BG, Jo EM, Kim GY, Yu BC, Hong MG, et al. The relationship between the fish consumption and blood total/methyl-mercury concentration of costal area in Korea. Neurotoxicology 2012;33(4):676–682. 22525937.ArticlePubMed
- 2. Kim JA, Yuk DH, Park Y, Choi HJ, Kim YC, Kim MS. A study on total mercury and methylmercury in commercial tuna, billfish, and deep-sea fish in Seoul metropolitan city. Korean J Food Sci Technol 2013;45(3):376–381.Article
- 3. Kim MK, Zoh KD. Fate and transport of mercury in environmental media and human exposure. J Prev Med Public Health 2012;45(6):335–343. 23230463.ArticlePubMedPMC
- 4. Choi W, Kim S, Baek YW, Choi K, Lee K, Kim S, et al. Exposure to environmental chemicals among Korean adults-updates from the second Korean National Environmental Health Survey (2012–2014). Int J Hyg Environ Health 2017;220(2 Pt A):29–35.Article
- 5. Yoshizawa K, Rimm EB, Morris JS, Spate VL, Hsieh CC, Spiegelman D, et al. Mercury and the risk of coronary heart disease in men. N Engl J Med 2002;347(22):1755–1760. 12456851.ArticlePubMed
- 6. Choi AL, Weihe P, Budtz-Jørgensen E, Jørgensen PJ, Salonen JT, Tuomainen TP, et al. Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environ Health Perspect 2009;117(3):367–372. 19337510.ArticlePubMedPMC
- 7. Houston MC. Role of mercury toxicity in hypertension, cardiovascular disease, and stroke. J Clin Hypertens (Greenwich) 2011;13(8):621–627. 21806773.ArticlePubMedPMC
- 8. Salonen JT, Seppänen K, Lakka TA, Salonen R, Kaplan GA. Mercury accumulation and accelerated progression of carotid atherosclerosis: a population-based prospective 4-year follow-up study in men in eastern Finland. Atherosclerosis 2000;148(2):265–273. 10657561.ArticlePubMed
- 9. Wiggers GA, Peçanha FM, Briones AM, Pérez-Girón JV, Miguel M, Vassallo DV, et al. Low mercury concentrations cause oxidative stress and endothelial dysfunction in conductance and resistance arteries. Am J Physiol Heart Circ Physiol 2008;295(3):H1033–H1043. 18599595.ArticlePubMed
- 10. Shin HY, Lee JY, Kim JE, Lee S, Youn H, Kim H, et al. Cause-of-death statistics in 2016 in the Republic of Korea. J Korean Med Assoc 2018;61(9):573–584.ArticlePDF
- 11. Yusuf S, Reddy S, Ôunpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 2001;104(22):2746–2753. 11723030.ArticlePubMed
- 12. Khot UN, Khot MB, Bajzer CT, Sapp SK, Ohman EM, Brener SJ, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA 2003;290(7):898–904. 12928466.ArticlePubMed
- 13. Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 1989;320(14):915–924. 2648148.ArticlePubMed
- 14. Salonen JT, Seppänen K, Nyyssönen K, Korpela H, Kauhanen J, Kantola M, et al. Intake of mercury from fish, lipid peroxidation, and the risk of myocardial infarction and coronary, cardiovascular, and any death in eastern Finnish men. Circulation 1995;91(3):645–655. 7828289.ArticlePubMed
- 15. You CH, Kim BG, Kim JM, Yu SD, Kim YM, Kim RB, et al. Relationship between blood mercury concentration and waist-to-hip ratio in elderly Korean individuals living in coastal areas. J Prev Med Public Health 2011;44(5):218–225. 22020187.ArticlePubMedPMC
- 16. Lim S, Choi MC, Joh KO, Paek D. The health effects of mercury on the cardiac autonomic activity according to the heart rate variability. Korean J Occup Environ Med 2008;20(4):302–313.ArticlePDF
- 17. Cho HW, Kim SH, Park MJ. An association of blood mercury levels and hypercholesterolemia among Korean adolescents. Sci Total Environ 2020;709:135965. 31927427.ArticlePubMed
- 18. Oh JS, Lee SH. Pb, Hg and Cd concentration of blood and exposure-related factors. J Korea Acad Ind Coop Soc 2015;16(3):2089–2099.Article
- 19. Lee BK, Kim Y. Blood cadmium, mercury, and lead and metabolic syndrome in South Korea: 2005–2010 Korean National Health and Nutrition Examination Survey. Am J Ind Med 2013;56(6):682–692. 22911659.ArticlePubMed
- 20. Yoo JY, Kim SY, Kwon YM, Jung SK, Lee CW, Yoo SD. The third Korean National Environmental Health Survey manual for the analysis of environmentally harmful substances in biological samples: heavy metals. Incheon: National Institute of Environmental Research; 2018.
- 21. Yoo JY, Kim SY, Hong SY, Lee CW. The third Korean National Environmental Health Survey manual for the analysis of laboratory tests in biological samples. Incheon: National Institute of Environmental Research; 2019.
- 22. Yoo JY, Kim SY, Joo YK, Jeon HR, Lee CW. The third Korean National Environmental Health Survey guidelines for using raw data (revised). Incheon: National Institute of Environmental Research; 2019.
- 23. Shin JY, Kim JM, Kim Y. The association of heavy metals in blood, fish consumption frequency, and risk of cardiovascular diseases among Korean adults: the Korean National Health and Nutrition Examination Survey (2008–2010). Korean J Nutr 2012;45(4):347–361.Article
- 24. Lee JW, Lee CK, Moon CS, Choi IJ, Lee KJ, Yi SM, et al. Korea national survey for environmental pollutants in the human body 2008: heavy metals in the blood or urine of the Korean population. Int J Hyg Environ Health 2012;215(4):449–457. 22341685.ArticlePubMed
- 25. Centers for Disease Control and Prevention. Fourth national report on human exposure to environmental chemicals. 2013;Accessed 10 October 2019]. http://www.cdc.gov/exposurereport/.
- 26. Kim DS, Lee EH, Yu SD, Cha JH, Ahn SC. Heavy metal as risk factor of cardiovascular disease--an analysis of blood lead and urinary mercury. J Prev Med Public Health 2005;38(4):401–407. 16358824.PubMed
- 27. Grotto D, de Castro MM, Barcelos GR, Garcia SC, Barbosa F Jr. Low level and sub-chronic exposure to methylmercury induces hypertension in rats: nitric oxide depletion and oxidative damage as possible mechanisms. Arch Toxicol 2009;83(7):653–662. 19468715.ArticlePubMedPDF
- 28. Lin TH, Huang YL, Huang SF. Lipid peroxidation in liver of rats administrated with methyl mercuric chloride. Biol Trace Elem Res 1996;54(1):33–41. 8862759.ArticlePubMedPDF
- 29. Carvalho CM, Chew EH, Hashemy SI, Lu J, Holmgren A. Inhibition of the human thioredoxin system. A molecular mechanism of mercury toxicity. J Biol Chem 2008;283(18):11913–11923. 18321861.PubMed
- 30. Khan MAK, Wang F. Mercury-selenium compounds and their toxicological significance: toward a molecular understanding of the mercury-selenium antagonism. Environ Toxicol Chem 2009;28(8):1567–1577. 19374471.ArticlePubMed
- 31. Shih DM, Xia YR, Wang XP, Miller E, Castellani LW, Subbanagounder G, et al. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J Biol Chem 2000;275(23):17527–17535. 10748217.ArticlePubMed
- 32. Ayotte P, Carrier A, Ouellet N, Boiteau V, Abdous B, Sidi EA, et al. Relation between methylmercury exposure and plasma paraoxonase activity in inuit adults from Nunavik. Environ Health Perspect 2011;119(8):1077–1083. 21543280.ArticlePubMedPMC
- 33. Pollack AZ, Sjaarda L, Ahrens KA, Mumford SL, Browne RW, Wactawski-Wende J, et al. Association of cadmium, lead and mercury with paraoxonase 1 activity in women. PLoS One 2014;9(3):e92152. 24682159.ArticlePubMedPMC
- 34. Gonzalvo MC, Gil F, Hernández AF, Villanueva E, Pla A. Inhibition of paraoxonase activity in human liver microsomes by exposure to EDTA, metals and mercurials. Chem Biol Interact 1997;105(3):169–179. 9291995.ArticlePubMed
- 35. Gençer N, Arslan O. Purification human PON1Q192 and PON1R192 isoenzymes by hydrophobic interaction chromatography and investigation of the inhibition by metals. J Chromatogr B Analyt Technol Biomed Life Sci 2009;877(3):134–140.ArticlePubMed
- 36. Costa LG, Vitalone A, Cole TB, Furlong CE. Modulation of paraoxonase (PON1) activity. Biochem Pharmacol 2005;69(4):541–550. 15670573.ArticlePubMed
- 37. Haines DA, Saravanabhavan G, Werry K, Khoury C. An overview of human biomonitoring of environmental chemicals in the Canadian Health Measures Survey: 2007–2019. Int J Hyg Environ Health 2017;220(2 Pt A):13–28. 27601095.ArticlePubMed
- 38. Schulz C, Wilhelm M, Heudorf U, Kolossa-Gehring M. Human Biomonitoring Commission of the German Federal Environment Agency. Update of the reference and HBM values derived by the German Human Biomonitoring Commission. Int J Hyg Environ Health 2011;215(1):26–35. 21820957.ArticlePubMed
- 39. Seo JW, Kim BG, Kim YM, Kim RB, Chung JY, Lee KM, et al. Trend of blood lead, mercury, and cadmium levels in Korean population: data analysis of the Korea National Health and Nutrition Examination Survey. Environ Monit Assess 2015;187(3):146. 25716526.ArticlePubMedPDF
- 40. Hightower JM, O'Hare A, Hernandez GT. Blood mercury reporting in NHANES: identifying Asian, Pacific Islander, Native American, and multiracial groups. Environ Health Perspect 2006;114(2):173–175. 16451850.ArticlePubMed
- 41. Kim YA, Kim YN, Cho KD, Kim MY, Kim EJ, Baek OH, et al. Blood heavy metal concentrations of Korean adults by seafood consumption frequency: using the fourth Korea National Health and Nutrition Examination Survey (KNHANES IV), 2008. Korean J Nutr 2011;44(6):518–526.Article
- 42. Liu Y, Buchanan S, Anderson HA, Xiao Z, Persky V, Turyk ME. Association of methylmercury intake from seafood consumption and blood mercury level among the Asian and Non-Asian populations in the United States. Environ Res 2018;160:212–222. 29020643.ArticlePubMed
- 43. Ilmiawati C, Yoshida T, Itoh T, Nakagi Y, Saijo Y, Sugioka Y, et al. Biomonitoring of mercury, cadmium, and lead exposure in Japanese children: a cross-sectional study. Environ Health Prev Med 2015;20(1):18–27. 25293698.ArticlePubMedPMCPDF
- 44. Eun JK, Lee WS. Mercury contents of human scalp hair by the consumption pattern in fish. Korean J Sanit 2000;15(3):8–14.
- 45. Vupputuri S, Longnecker MP, Daniels JL, Guo X, Sandler DP. Blood mercury level and blood pressure among US women: results from the National Health and Nutrition Examination Survey 1999–2000. Environ Res 2005;97(2):195–200. 15533335.ArticlePubMed
- 46. Wennberg M, Bergdahl IA, Stegmayr B, Hallmans G, Lundh T, Skerfving S, et al. Fish intake, mercury, long-chain n-3 polyunsaturated fatty acids and risk of stroke in northern Sweden. Br J Nutr 2007;98(5):1038–1045. 17537290.ArticlePubMed
- 47. Mozaffarian D, Longstreth WT Jr, Lemaitre RN, Manolio TA, Kuller LH, Burke GL, et al. Fish consumption and stroke risk in elderly individuals: the cardiovascular health study. Arch Intern Med 2005;165(2):200–206. 15668367.ArticlePubMedPMC
- 48. Iso H, Rexrode KM, Stampfer MJ, Manson JE, Colditz GA, Speizer FE, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA 2001;285(3):304–312. 11176840.ArticlePubMed
- 49. Kris-Etherton PM, Harris WS, Appel LJ. American Heart Association Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002;106(21):2747–2757. 12438303.ArticlePubMed
- 50. König A, Bouzan C, Cohen JT, Connor WE, Kris-Etherton PM, Gray GM, et al. A quantitative analysis of fish consumption and coronary heart disease mortality. Am J Prev Med 2005;29(4):335–346. 16242600.ArticlePubMed
- 51. Ström S, Helmfrid I, Glynn A, Berglund M. Nutritional and toxicological aspects of seafood consumption--an integrated exposure and risk assessment of methylmercury and polyunsaturated fatty acids. Environ Res 2011;111(2):274–280. 21211794.ArticlePubMed
- 52. Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E. Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr 2006;84(4):762–773. 17023702.ArticlePubMedPMC
- 53. Park K, Seo E. Toenail mercury and dyslipidemia: Interaction with selenium. J Trace Elem Med Biol 2017;39:43–49. 27908422.ArticlePubMed
- 54. Park K, Seo E. Association between toenail mercury and metabolic syndrome is modified by selenium. Nutrients 2016;8(7):E424. 27420091.ArticlePubMedPMC
- 55. Hong D, Cho SH, Park SJ, Kim SY, Park SB. Hair mercury level in smokers and its influence on blood pressure and lipid metabolism. Environ Toxicol Pharmacol 2013;36(1):103–107. 23603462.ArticlePubMed
REFERENCES
REFERENCES
Figure & Data
REFERENCES
Citations

- Re-thinking the link between exposure to mercury and blood pressure
Xue Feng Hu, Allison Loan, Hing Man Chan
Archives of Toxicology.2025; 99(2): 481. CrossRef - Do blood metals influence lipid profiles? Findings of a cross-sectional population-based survey
Sabit Cakmak, Kimberly Mitchell, Anna Lukina, Robert Dales
Environmental Research.2023; 231: 116107. CrossRef - Association of Blood Total Mercury with Dyslipidemia in a sample of U.S. Adolescents: Results from the National Health and Nutrition Examination Survey Database, 2011–2018
Chibuzor Abasilim, Victoria Persky, Mary E. Turyk
Hygiene and Environmental Health Advances.2023; 6: 100047. CrossRef - Association of Blood Mercury Level with Liver Enzymes in Korean Adults: An Analysis of 2015–2017 Korean National Environmental Health Survey
Jin-Wook Chung, Dilaram Acharya, Jitendra Kumar Singh, Joon Sakong
International Journal of Environmental Research and Public Health.2023; 20(4): 3290. CrossRef - Heavy metal-induced lipogenic gene aberration, lipid dysregulation and obesogenic effect: a review
Yang Zhou, Frank Peprah Addai, Xinshuang Zhang, Yuelin Liu, Yinfeng Wang, Feng Lin, Alex Tuffour, Jie Gu, Guangxiang Liu, Haifeng Shi
Environmental Chemistry Letters.2022; 20(3): 1611. CrossRef - Mercury may reduce the protective effect of sea fish consumption on serum triglycerides levels in Chinese adults: Evidence from China National Human Biomonitoring
Bing Wu, Yingli Qu, Yifu Lu, Saisai Ji, Liang Ding, Zheng Li, Miao Zhang, Heng Gu, Qi Sun, Bo Ying, Feng Zhao, Xulin Zheng, Yidan Qiu, Zheng Zhang, Ying Zhu, Zhaojin Cao, Yuebin Lv, Xiaoming Shi
Environmental Pollution.2022; 311: 119904. CrossRef - Misuse of Cardiac Lipid upon Exposure to Toxic Trace Elements—A Focused Review
Kaviyarasi Renu, Anirban Goutam Mukherjee, Uddesh Ramesh Wanjari, Sathishkumar Vinayagam, Vishnu Priya Veeraraghavan, Balachandar Vellingiri, Alex George, Ricardo Lagoa, Kamaraj Sattu, Abhijit Dey, Abilash Valsala Gopalakrishnan
Molecules.2022; 27(17): 5657. CrossRef - The effects of chemical mixtures on lipid profiles in the Korean adult population: threshold and molecular mechanisms for dyslipidemia involved
Hai Duc Nguyen, Hojin Oh, Min-Sun Kim
Environmental Science and Pollution Research.2022; 29(26): 39182. CrossRef - Association between Heavy Metal Exposure and Dyslipidemia among Korean Adults: From the Korean National Environmental Health Survey, 2015–2017
Do-won Kim, Jeongwon Ock, Kyong-Whan Moon, Choong-Hee Park
International Journal of Environmental Research and Public Health.2022; 19(6): 3181. CrossRef
- Figure
- Related articles
-
- Relationship between the use of hair products and urine benzophenone-3: the Korean National Environmental Health Survey (KoNEHS) cycle 4
- Relationship between crustacean consumption and serum perfluoroalkyl substances (PFAS): the Korean National Environmental Health Survey (KoNEHS) cycle 4
- Association between serum perfluoroalkyl substances concentrations and non-alcoholic fatty liver disease among Korean adults: a cross-sectional study using the National Environmental Health Survey cycle 4
- Relationship between the use of plastics in refrigerator food storage and urine phthalate metabolites: the Korean National Environmental Health Survey (KoNEHS) cycle 3
- The association of perfluoroalkyl substances (PFAS) exposure and kidney function in Korean adolescents using data from Korean National Environmental Health Survey (KoNEHS) cycle 4 (2018–2020): a cross-sectional study
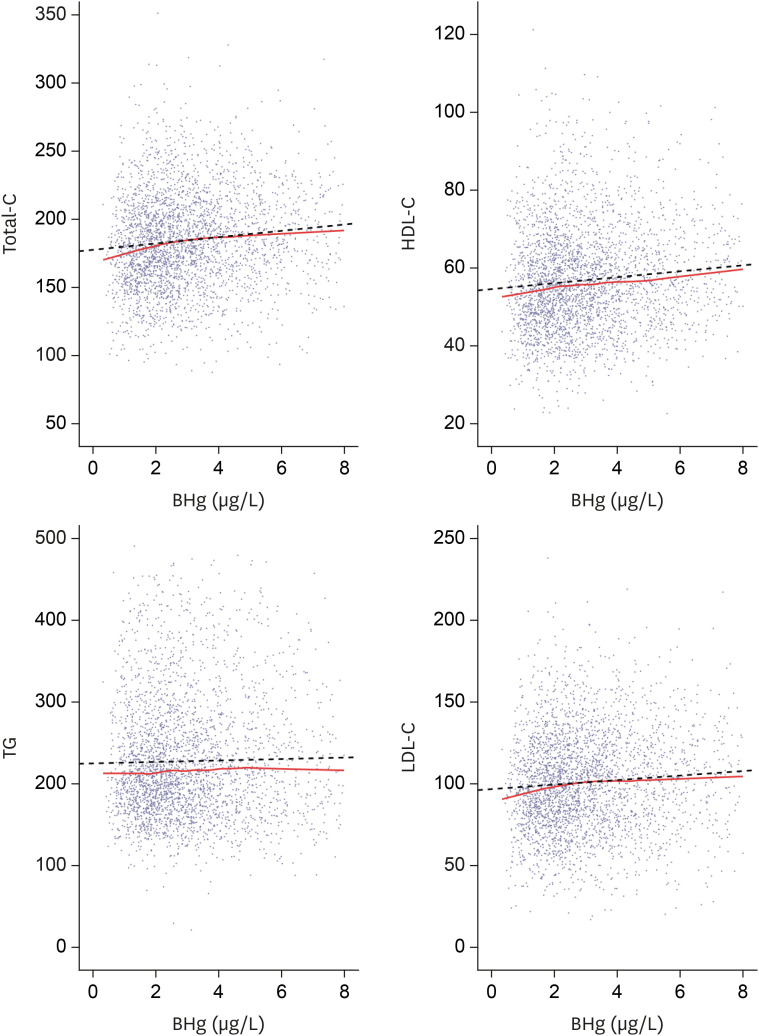
Fig. 1
| Variables | Total | Males | Females | |
|---|---|---|---|---|
| Total (%) | 3,228 (100) | 1,400 (43.4) | 1,828 (56.6) | |
| Age (years) | ||||
| 19–29 | 268 (8.3) | 128 (9.1) | 140 (7.7) | |
| 30–39 | 505 (15.6) | 201 (14.4) | 304 (16.6) | |
| 40–49 | 568 (17.6) | 240 (17.1) | 328 (17.9) | |
| 50–59 | 744 (23.0) | 292 (20.9) | 452 (24.7) | |
| 60–69 | 726 (22.5) | 342 (24.4) | 384 (21.0) | |
| ≥ 70 | 417 (12.9) | 197 (14.1) | 220 (12.0) | |
| BMI (kg/m2) | ||||
| < 23 | 1,147 (35.5) | 418 (29.9) | 729 (39.9) | |
| ≥ 23 and < 25 | 838 (26.0) | 384 (27.4) | 454 (24.8) | |
| ≥ 25 | 1,243 (38.5) | 598 (42.7) | 645 (35.3) | |
| Smoking | ||||
| Never smoked | 2,088 (64.7) | 348 (24.9) | 1,740 (95.2) | |
| Past smokers | 639 (19.7) | 598 (42.7) | 39 (2.1) | |
| Current smokers | 503 (15.6) | 454 (32.4) | 49 (2.7) | |
| Drinking | ||||
| Yes | 2,600 (80.5) | 1,278 (91.3) | 1,322 (72.3) | |
| No | 628 (19.5) | 122 (8.7) | 506 (27.7) | |
| Education | ||||
| High school graduation or less | 2,177 (67.4) | 896 (64.0) | 1,281 (70.1) | |
| College graduation or more | 1,051 (32.6) | 504 (36.0) | 547 (29.9) | |
| Variables | No. | BHg (µg/L) | UHg (µg/L) | |||
|---|---|---|---|---|---|---|
| GM (95% CI) | AM (95% CI) | GM (95% CI) | AM (95% CI) | |||
| Total | 3,228 | 2.71 (2.59–2.85) | 2.91 (2.81–3.02) | 0.35 (0.33–0.38) | 0.52 (0.48–0.56) | |
| Sex | ||||||
| Male | 1,400 | 3.23 (3.01–3.47) | 4.14 (3.86–4.42) | 0.40 (0.37–0.43) | 0.57 (0.52–0.62) | |
| Female | 1,828 | 2.29 (2.19–2.40) | 2.73 (2.59–2.87) | 0.32 (0.30–0.34) | 0.47 (0.43–0.52) | |
| p-value | < 0.001 | < 0.001 | < 0.001 | 0.002 | ||
| Age (years) | ||||||
| 19–29 | 268 | 1.91 (1.72–2.13) | 2.26 (2.04–2.48) | 0.33 (0.29–0.38) | 0.49 (0.38–0.59) | |
| 30–39 | 505 | 2.87 (2.66–3.10) | 3.51 (3.19–3.83) | 0.40 (0.36–0.45) | 0.58 (0.51–0.65) | |
| 40–49 | 568 | 2.96 (2.78–3.16) | 3.62 (3.34–3.90) | 0.37 (0.34–0.40) | 0.54 (0.47–0.61) | |
| 50–59 | 744 | 3.30 (3.09–3.52) | 4.17 (3.78–4.56) | 0.38 (0.35–0.42) | 0.55 (0.49–0.61) | |
| 60–69 | 726 | 3.07 (2.82–3.34) | 3.93 (3.56–4.30) | 0.33 (0.30–0.36) | 0.48 (0.42–0.54) | |
| ≥ 70 | 417 | 2.39 (2.14–2.69) | 3.08 (2.67–3.49) | 0.28 (0.25–0.32) | 0.41 (0.35–0.47) | |
| p-value | < 0.001 | < 0.001 | < 0.001 | 0.001 | ||
| BMI (kg/m2) | ||||||
| < 23 | 1,147 | 2.29 (2.16–2.44) | 2.83 (2.65–3.00) | 0.32 (0.30–0.34) | 0.48 (0.43–0.53) | |
| ≥ 23 and < 25 | 838 | 2.94 (2.75–3.14) | 3.67 (3.39–3.95) | 0.35 (0.32–0.38) | 0.49 (0.44–0.54) | |
| ≥ 25 | 1,243 | 3.09 (2.92–3.27) | 3.91 (3.63–4.18) | 0.40 (0.37–0.44) | 0.59 (0.52–0.65) | |
| p-value | < 0.001 | < 0.001 | < 0.001 | 0.008 | ||
| Smoking | ||||||
| Never smoked | 2,088 | 2.37 (2.26–2.48) | 2.64 (2.53–2.74) | 0.33 (0.31–0.35) | 0.47 (0.43–0.51) | |
| Past smoker | 637 | 3.56 (3.32–3.82) | 3.53 (3.31–3.74) | 0.42 (0.38–0.46) | 0.62 (0.55–0.70) | |
| Current smoker | 503 | 3.29 (2.97–3.65) | 3.32 (3.06–3.57) | 0.40 (0.36–0.45) | 0.59 (0.50–0.68) | |
| p-value | < 0.001 | < 0.001 | < 0.001 | 0.003 | ||
| Drinking | ||||||
| Yes | 2,600 | 2.77 (2.27–2.60) | 3.50 (3.31–3.69) | 0.36 (0.34–0.39) | 0.54 (0.49–0.58) | |
| No | 628 | 2.43 (2.27–2.60) | 2.94 (2.72–3.15) | 0.31 (0.29–0.34) | 0.43 (0.38–0.47) | |
| p-value | < 0.001 | < 0.001 | 0.001 | < 0.001 | ||
| Education | ||||||
| High school graduation or less | 2,177 | 2.61 (2.45–2.78) | 3.32 (3.09–3.55) | 0.34 (0.32–0.37) | 0.49 (0.45–0.54) | |
| College graduation or more | 1,051 | 2.87 (2.71–3.02) | 3.56 (3.31–3.80) | 0.37 (0.35–0.40) | 0.56 (0.50–0.62) | |
| p-value | 0.012 | < 0.001 | 0.050 | 0.274 | ||
| Variables | Total-C | HDL-C | TG | LDL-C | |
|---|---|---|---|---|---|
| Blood mercury levels in males | |||||
| Q1 (0.430–2.249) (n = 350) | 173.25 ± 3.57 | 50.61 ± 0.88 | 158.01 ± 5.91 | 91.03 ± 3.25 | |
| Q2 (2.249–3.437) (n = 350) | 183.97 ± 2.25 | 52.90 ± 1.10 | 165.70 ± 4.74 | 97.93 ± 2.14 | |
| Q3 (3.437–5.437) (n = 350) | 186.88 ± 2.27 | 52.21 ± 0.80 | 163.77 ± 5.90 | 101.91 ± 2.12 | |
| Q4 (5.437+) (n = 350) | 193.06 ± 2.03 | 54.28 ± 0.99 | 174.66 ± 5.57 | 103.84 ± 1.94 | |
| p-value | < 0.001 | 0.029 | 0.261 | 0.006 | |
| p for trend | < 0.001 | 0.013 | 0.074 | 0.001 | |
| Urine mercury levels in males | |||||
| Q1 (0.070–0.220) (n = 352) | 174.31 ± 3.11 | 52.27 ± 1.00 | 146.76 ± 5.65 | 92.69 ± 2.87 | |
| Q2 (0.220–0.390) (n = 356) | 181.40 ± 2.34 | 52.85 ± 0.84 | 176.51 ± 6.16 | 93.25 ± 2.29 | |
| Q3 (0.390–0.710) (n = 348) | 188.80 ± 2.77 | 52.13 ± 0.78 | 168.63 ± 5.00 | 102.94 ± 2.67 | |
| Q4 (0.710+) (n = 344) | 190.32 ± 2.35 | 52.29 ± 1.18 | 167.79 ± 5.84 | 104.47 ± 2.05 | |
| p-value | < 0.001 | 0.931 | 0.002 | < 0.001 | |
| P for trend | < 0.001 | 0.887 | 0.042 | < 0.001 | |
| Blood mercury levels in females | |||||
| Q1 (0.330–1.690) (n = 457) | 180.60 ± 2.13 | 60.65 ± 0.79 | 124.45 ± 4.15 | 95.06 ± 1.99 | |
| Q2 (1.690–2.402) (n = 457) | 183.81 ± 1.88 | 62.20 ± 0.97 | 128.64 ± 4.58 | 95.89 ± 1.56 | |
| Q3 (2.402–3.593) (n = 457) | 188.29 ± 2.17 | 60.19 ± 0.85 | 136.72 ± 4.38 | 100.75 ± 1.78 | |
| Q4 (3.593+) (n = 457) | 191.69 ± 1.98 | 61.95 ± 1.21 | 143.10 ± 4.67 | 101.12 ± 1.90 | |
| p-value | 0.001 | 0.327 | 0.018 | 0.019 | |
| p for trend | < 0.001 | 0.654 | 0.002 | 0.009 | |
| Urine mercury levels in females | |||||
| Q1 (0.070–0.190) (n = 491) | 183.45 ± 1.85 | 62.08 ± 0.80 | 133.67 ± 4.31 | 94.64 ± 1.78 | |
| Q2 (0.190–0.320) (n = 442) | 185.91 ± 2.21 | 61.37 ± 1.00 | 130.75 ± 4.02 | 98.39 ± 1.91 | |
| Q3 (0.320–0.580) (n = 448) | 185.45 ± 1.86 | 59.95 ± 1.01 | 135.27 ± 4.56 | 98.44 ± 1.62 | |
| Q4 (0.580+) (n = 447) | 187.94 ± 2.06 | 61.23 ± 1.03 | 129.34 ± 3.96 | 100.84 ± 1.72 | |
| p-value | 0.506 | 0.402 | 0.766 | 0.123 | |
| p for trend | 0.182 | 0.280 | 0.654 | 0.025 | |
| Variables | Total-C | HDL-C | TG | LDL-C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | p-value | β | SE | p-value | β | SE | p-value | β | SE | p-value | |
| Age | 0.142 | 0.063 | 0.025 | −0.086 | 0.023 | < 0.001 | 0.369 | 0.128 | 0.005 | 0.154 | 0.053 | 0.004 |
| Sex | 9.314 | 3.109 | 0.003 | 9.885 | 0.803 | < 0.001 | −19.518 | 4.540 | < 0.001 | 3.333 | 2.932 | 0.257 |
| Smoking | 4.257 | 3.034 | 0.162 | 1.203 | 1.005 | 0.233 | 7.792 | 5.146 | 0.132 | 1.496 | 2.753 | 0.587 |
| Alcohol | 1.897 | 2.078 | 0.363 | 4.069 | 0.724 | < 0.001 | −5.305 | 5.360 | 0.324 | −1.111 | 1.764 | 0.529 |
| Seafood intake | 0.796 | 2.889 | 0.783 | 1.130 | 0.895 | 0.208 | 0.893 | 5.858 | 0.879 | −0.513 | 2.279 | 0.822 |
| BMI | 1.091 | 0.250 | < 0.001 | −1.163 | 0.092 | < 0.001 | 7.537 | 0.530 | < 0.001 | 0.747 | 0.210 | < 0.001 |
| Education level | 2.869 | 1.788 | 0.110 | 1.152 | 0.719 | 0.111 | −1.813 | 3.867 | 0.640 | 2.080 | 1.637 | 0.206 |
| BHg (µg/L) | 2.927 | 0.580 | < 0.001 | 0.835 | 0.409 | < 0.001 | 0.647 | 1.082 | 0.551 | 1.962 | 0.509 | < 0.001 |
| Variables | No. | Total-C | HDL-C | TG | LDL-C | |
|---|---|---|---|---|---|---|
| HBM I | ||||||
| < 5 | 2,602 (80.6) | 182.95 ± 1.01 | 56.97 ± 0.46 | 144.98 ± 2.25 | 96.99 ± 0.91 | |
| ≥ 5 | 626 (19.4) | 192.82 ± 1.79 | 56.31 ± 0.92 | 165.11 ± 4.21 | 103.48 ± 1.62 | |
| p-value | < 0.001 | 0.502 | < 0.001 | < 0.001 | ||
| HBM II | ||||||
| < 15 | 3,178 (98.5) | 184.65 ± 0.94 | 56.89 ± 0.44 | 148.19 ± 2.10 | 98.13 ± 0.84 | |
| ≥ 15 | 50 (1.5) | 183.02 ± 6.47 | 53.59 ± 3.62 | 172.64 ± 16.71 | 94.90 ± 5.76 | |
| p-value | 0.805 | 0.368 | 0.153 | 0.578 | ||
| Frequency of fish and shellfish intake | BHg (µg/L) | |
|---|---|---|
| Fish | Shellfish | |
| Rarely | 2.22 ± 0.26 | 2.75 ± 0.11 |
| 1/month | 2.43 ± 0.12 | 3.11 ± 0.13 |
| 2–3/month | 2.90 ± 0.12 | 3.04 ± 0.16 |
| 1/week | 3.02 ± 0.11 | 3.47 ± 0.28 |
| ≥ 2/week | 3.67 ± 0.14 | 3.52 ± 0.27 |
| p-value | < 0.001 | 0.004 |
Values are presented as number (%).
BMI: body mass index.
BMI: body mass index; BHg: blood mercury concentrations; UHg: urine mercury concentrations; GM: geometric mean; CI: confidence interval; AM: arithmetic mean.
Data are shown as mean ± standard error (mg/dL).
Total-C: total cholesterol; HDL-C: high-density lipoprotein cholesterol; TG: triglyceride; LDL-C: low-density lipoprotein cholesterol.
Total-C: total cholesterol; HDL-C: high-density lipoprotein cholesterol; TG: triglyceride; LDL-C: low-density lipoprotein cholesterol; SE: standard error; BMI: body mass index; BHg: blood mercury concentrations.
Data are shown as mean ± standard error (mg/dL) or number (%).
HBM: human bio-monitoring; Total-C: total cholesterol; HDL-C: high-density lipoprotein cholesterol; TG: triglyceride; LDL-C: low-density lipoprotein-cholesterol.
Data are shown as mean ± standard error. Adjusted by age, sex, smoking and alcohol consumption.
BHg: blood mercury concentrations.
 KSOEM
KSOEM

 Cite
Cite

