Articles
- Page Path
- HOME > Ann Occup Environ Med > Volume 31; 2019 > Article
- Research Article Association between urinary phthalate metabolites and obesity in adult Korean population: Korean National Environmental Health Survey (KoNEHS), 2012–2014
-
Yangwon Kang1
 , Juha Park2
, Juha Park2 , Kanwoo Youn3
, Kanwoo Youn3
-
Annals of Occupational and Environmental Medicine 2019;31:e23.
DOI: https://doi.org/10.35371/aoem.2019.31.e23
Published online: September 9, 2019
1Department of Occupational and Environmental Medicine, Inha University Hospital, Incheon, Korea.
2Department of Occupational and Environmental Medicine, Dankook University Hospital, Cheonan, Korea.
3Department of Occupational and Environmental Medicine, Wonjin Green Hospital, Seoul, Korea.
- Correspondence: Kanwoo Youn. Department of Occupational and Environmental Medicine, Wonjin Green Hospital, 568-1 Sagajeong-ro 49-gil 53, Jungrang-gu, Seoul 02221, Korea. younkw76@gmail.com
Copyright © 2019 Korean Society of Occupational & Environmental Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background Phthalate is a chemical that is commonly used as a plasticizer in processing plastic products and as a solvent in personal care products. Although previous experimental studies have reported that phthalate metabolites are associated with obesity, epidemiological study results have been inconsistent and insufficient. The objective of the present study was to investigate the association between urinary phthalate metabolites and obesity in adult Korean population.
-
Methods The present study selected 4,752 Korean adults aged 19 years or older from the 2012–2014 Korean National Environmental Health Survey data. The concentrations of urinary di-(2-ethyl-5-carboxypentyl) phthalate (DEHP) metabolites—i.e., mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP) and mono-(2-ethyl-5-carboxypentyl) phthalate—mono-benzyl phthalate (MBzP) and mono-n-butyl phthalate (MnBP) were adjusted using the urinary creatinine. We used logistic regression analysis to investigate the association between urinary phthalate metabolite concentration and body mass index (BMI) with respect to sex and age.
-
Results Among women, urinary MEHHP and DEHP concentrations were found to have statistically significantly positive associations with obesity (Q4 versus Q1; odds ratio (OR): 1.72, 95% confidence interval (CI): 1.19–2.49 for MEHHP and OR: 1.52, 95% CI: 1.04–2.21 for DEHP). Among men, urinary MnBP concentration was found to have statistically significantly negative association with obesity (Q4 versus Q1; OR: 0.71, 95% CI: 0.50–0.99). In the analysis stratified by sex and age, women aged ≥ 50 years showed statistically significantly positive associations between the concentrations of urinary DEHP metabolites, DEHP, MBzP, and obesity (Q4 versus Q1; OR: 1.94, 95% CI: 1.28–2.94 for MEHHP, OR: 1.88, 95% CI: 1.21–2.94 for MEOHP, OR: 2.04, 95% CI: 1.31–3.18 for DEHP, and Q3 versus Q1; OR: 1.45, 95% CI: 1.02–2.05 for MBzP). Meanwhile, men aged ≥ 50 years showed no significant associations between urinary phthalate concentrations and obesity.
-
Conclusions In the present study, we found differences in the associations between urinary phthalate metabolites and BMI according to sex and age. However, because the present study was cross-sectional in nature, additional support through prospective studies is needed to estimate the causal associations.
BACKGROUND
METHODS
RESULTS
Demographic distributions of the study subjects according to characteristics
Weighted percentages of body mass index groups by characteristics
Geometric mean of urinary phthalate metabolites (μg/g creatinine) by categories of body mass index
Odds ratios and 95% confidence intervals for obesity (body mass index > 30 kg/m2) according to urinary phthalate metabolites in men and women
Adjusted OR and 95% confidence intervals for obesity (body mass index > 30 kg/m2) according to urinary phthalate metabolites stratified by age and sex
DISCUSSION
CONCLUSIONS
Acknowledgements
Abbreviations
BBzP
BMI
CI
DBP
DEHP
EDC
GM
HMW
KoNEHS
LOD
MBzP
MCNP
MECPP
MEHHP
MEHP
MEOHP
MEP
MnBP
NHANES
OR
PPARs
TSH
T4
-
Competing interests: The authors declare that they have no competing interests.
-
Author Contributions:
NOTES
- 1. Oh SW. Obesity and metabolic syndrome in Korea. Diabetes Metab J 2011;35(6):561–566. 22247896.ArticlePubMedPMC
- 2. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348(17):1625–1638. 12711737.ArticlePubMed
- 3. Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin 2017;67(5):378–397. 28763097.ArticlePubMedPMCPDF
- 4. Shin HY, Kang HT. Recent trends in the prevalence of underweight, overweight, and obesity in Korean adults: The Korean National Health and Nutrition Examination Survey from 1998 to 2014. J Epidemiol 2017;27(9):413–419. 28420559.ArticlePubMedPMC
- 5. Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science 1998;280(5368):1371–1374. 9603719.ArticlePubMed
- 6. Heindel JJ, Newbold R, Schug TT. Endocrine disruptors and obesity. Nat Rev Endocrinol 2015;11(11):653–661. 26391979.ArticlePubMedPDF
- 7. Darbre PD. Endocrine disruptors and obesity. Curr Obes Rep 2017;6(1):18–27. 28205155.ArticlePubMedPMCPDF
- 8. Ventrice P, Ventrice D, Russo E, De Sarro G. Phthalates: European regulation, chemistry, pharmacokinetic and related toxicity. Environ Toxicol Pharmacol 2013;36(1):88–96. 23603460.ArticlePubMed
- 9. Rudel RA, Perovich LJ. Endocrine disrupting chemicals in indoor and outdoor air. Atmos Environ (1994) 2009;43(1):170–181. 20047015.ArticlePubMedPMC
- 10. Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res 2008;108(2):177–184. 18949837.ArticlePubMedPMC
- 11. Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, et al. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology 2012;153(9):4097–4110. 22733974.ArticlePubMedPMCPDF
- 12. Buser MC, Murray HE, Scinicariello F. Age and sex differences in childhood and adulthood obesity association with phthalates: analyses of NHANES 2007–2010. Int J Hyg Environ Health 2014;217(6):687–694. 24657244.ArticlePubMedPMC
- 13. Yaghjyan L, Sites S, Ruan Y, Chang SH. Associations of urinary phthalates with body mass index, waist circumference and serum lipids among females: National Health and Nutrition Examination Survey 1999–2004. Int J Obes (Lond) 2015;39(6):994–1000. 25644057.ArticlePubMedPMCPDF
- 14. Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, et al. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ Health 2008;7(1):27. 18522739.ArticlePubMedPMCPDF
- 15. Park C, Choi W, Hwang M, Lee Y, Kim S, Yu S, et al. Associations between urinary phthalate metabolites and bisphenol A levels, and serum thyroid hormones among the Korean adult population - Korean National Environmental Health Survey (KoNEHS) 2012–2014. Sci Total Environ 2017;584-585:950–957. 28153396.ArticlePubMed
- 16. Kim YT, Cha C, Lee MR. Factors related to age at menopause among Korean women: the Korean longitudinal survey of women and families. Menopause 2019;26(5):492–498. 30531439.ArticlePubMed
- 17. Takahashi TA, Johnson KM. Menopause. Med Clin North Am 2015;99(3):521–534. 25841598.ArticlePubMed
- 18. Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect 2007;115(6):876–882. 17589594.ArticlePubMedPMC
- 19. Díaz Santana MV, Hankinson SE, Bigelow C, Sturgeon SR, Zoeller RT, Tinker L, et al. Urinary concentrations of phthalate biomarkers and weight change among postmenopausal women: a prospective cohort study. Environ Health 2019;18(1):20. 30866962.PubMedPMC
- 20. Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes 2008;32(6):949–958.ArticlePubMedPMCPDF
- 21. Salpeter SR, Walsh JM, Ormiston TM, Greyber E, Buckley NS, Salpeter EE. Meta-analysis: effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes Metab 2006;8(5):538–554. 16918589.ArticlePubMed
- 22. Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect 2003;111(2):139–145. 12573895.ArticlePubMedPMC
- 23. Dubois V, Eeckhoute J, Lefebvre P, Staels B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J Clin Invest 2017;127(4):1202–1214. 28368286.ArticlePubMedPMC
- 24. Gross B, Pawlak M, Lefebvre P, Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol 2017;13(1):36–49. 27636730.ArticlePubMedPDF
- 25. Feige JN, Gelman L, Rossi D, Zoete V, Métivier R, Tudor C, et al. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J Biol Chem 2007;282(26):19152–19166. 17468099.PubMed
- 26. Hao C, Cheng X, Xia H, Ma X. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci Rep 2012;32(6):619–629. 22953781.ArticlePubMedPMCPDF
- 27. Knudsen N, Laurberg P, Rasmussen LB, Bülow I, Perrild H, Ovesen L, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab 2005;90(7):4019–4024. 15870128.ArticlePubMed
- 28. Blouin K, Boivin A, Tchernof A. Androgens and body fat distribution. J Steroid Biochem Mol Biol 2008;108(3-5):272–280. 17945484.ArticlePubMed
- 29. Fong JP, Lee FJ, Lu IS, Uang SN, Lee CC. Relationship between urinary concentrations of di(2-ethylhexyl) phthalate (DEHP) metabolites and reproductive hormones in polyvinyl chloride production workers. Occup Environ Med 2015;72(5):346–353. 25637220.ArticlePubMed
- 30. Wang YX, Zeng Q, Sun Y, You L, Wang P, Li M, et al. Phthalate exposure in association with serum hormone levels, sperm DNA damage and spermatozoa apoptosis: a cross-sectional study in China. Environ Res 2016;150:557–565. 26654563.ArticlePubMed
- 31. Meeker JD, Calafat AM, Hauser R. Urinary metabolites of di(2-ethylhexyl) phthalate are associated with decreased steroid hormone levels in adult men. J Androl 2009;30(3):287–297. 19059903.ArticlePubMedPMC
- 32. Shoaff J, Papandonatos GD, Calafat AM, Ye X, Chen A, Lanphear BP, et al. Early-life phthalate exposure and adiposity at 8 years of age. Environ Health Perspect 2017;125(9):097008. 28935615.ArticlePubMedPMC
- 33. Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect 2004;112(17):1734–1740. 15579421.ArticlePubMedPMC
REFERENCES
REFERENCES
Figure & Data
REFERENCES
Citations

- Associations between exposure to environmental pollutants, metabolic syndrome risk, and obesity-related anthropometric indices
Iman Al-Saleh, Rola Elkhatib, Reem Alswayeh, Reem Al-Rouqi, Mawadah Baali, Yara Aljerayed, Sophia S. De Padua, Hissah Alnuwaysir, Ghada Hussein, Habiba Sultana, Naveed Yousaf, Abass Waqar, Khalid Alhusayn, Shoaib Khan, Amber Shammama, Abdullah Aldowaish,
International Journal of Hygiene and Environmental Health.2026; 272: 114720. CrossRef - Associations of phthalate and phthalate alternative metabolites in urine with the risk of gallstones in adults: a cross-sectional analysis
Tianshan Shi, Di Li, Donghua Li, Peng Xie, Jin Sun, Tingrong Wang, Rui Li, Zixuan Zou, Zhenjuan Li, Xiaowei Ren
Environmental Geochemistry and Health.2025;[Epub] CrossRef - Phthalate Metabolites and Their Relationship with Abdominal and General Obesity: Evidence from the Aragon Workers’ Health Study (AWHS)
Jordan Akritidis, Diana María Mérida, Carolina Torrijo-Belanche, Belén Moreno-Franco, Sofía Gimeno-Ruiz, Jimena Rey-García, María Morales-Suarez-Varela, Pilar Guallar-Castillón
Nutrients.2025; 17(11): 1869. CrossRef - Gender-specific abdominal fat distribution and insulin resistance associated with organophosphate esters and phthalate metabolites exposure
Xiaoliu Shi, Wanyue Wang, Jiafan Feng, Xiaochun Ma, Mengting Xu, Cui Wang
Environmental Pollution.2024; 349: 123959. CrossRef - Phthalates’ exposure leads to an increasing concern on cardiovascular health
Melissa Mariana, Miguel Castelo-Branco, Amadeu M. Soares, Elisa Cairrao
Journal of Hazardous Materials.2023; 457: 131680. CrossRef - Association between phthalate exposure and obesity risk: A meta-analysis of observational studies
Qian Wu, Gang Li, Chen-Yang Zhao, Xiao-Lin Na, Yun-Bo Zhang
Environmental Toxicology and Pharmacology.2023; 102: 104240. CrossRef - Risk of Nonalcoholic Fatty Liver Disease Is Associated with Urinary Phthalate Metabolites Levels in Adults with Subclinical Hypothyroidism: Korean National Environmental Health Survey (KoNEHS) 2012–2014
Eun-Jung Yang, Byung-Sun Choi, Yun-Jung Yang
International Journal of Environmental Research and Public Health.2022; 19(6): 3267. CrossRef - The effects of chemical mixtures on lipid profiles in the Korean adult population: threshold and molecular mechanisms for dyslipidemia involved
Hai Duc Nguyen, Hojin Oh, Min-Sun Kim
Environmental Science and Pollution Research.2022; 29(26): 39182. CrossRef - Association of Exposure to Phthalate Metabolites With Sex Hormones, Obesity, and Metabolic Syndrome in US Women
Pallavi Dubey, Sireesha Y. Reddy, Vishwajeet Singh, Ted Shi, Mallorie Coltharp, Deborah Clegg, Alok K. Dwivedi
JAMA Network Open.2022; 5(9): e2233088. CrossRef - Mixtures modeling identifies heavy metals and pyrethroid insecticide metabolites associated with obesity
Hai Duc Nguyen, Hojin Oh, Won Hee Jo, Ngoc Hong Minh Hoang, Min-Sun Kim
Environmental Science and Pollution Research.2022; 29(14): 20379. CrossRef - Life-Time Environmental Chemical Exposure and Obesity: Review of Epidemiological Studies Using Human Biomonitoring Methods
Nayan Chandra Mohanto, Yuki Ito, Sayaka Kato, Michihiro Kamijima
Frontiers in Endocrinology.2021;[Epub] CrossRef - Relationships between di-(2-ethylhexyl) phthalate exposure and lipid metabolism in adolescents: Human data and experimental rat model analyses
Shuang Ding, Wen Qi, Qi Xu, Tianyang Zhao, Xu Li, Jianli Yin, Ruxuan Zhang, Chuanyi Huo, Liting Zhou, Lin Ye
Environmental Pollution.2021; 286: 117570. CrossRef - Association between Blood Mercury Levels and Non-Alcoholic Fatty Liver Disease in Non-Obese Populations: The Korean National Environmental Health Survey (KoNEHS) 2012–2014
Yun-Jung Yang, Eun-Jung Yang, Kyongjin Park, Subin Oh, Taehyen Kim, Yeon-Pyo Hong
International Journal of Environmental Research and Public Health.2021; 18(12): 6412. CrossRef - The association between urinary bisphenol A levels and nonalcoholic fatty liver disease in Korean adults: Korean National Environmental Health Survey (KoNEHS) 2015-2017
Sang Joon An, Eun-Jung Yang, Subin Oh, Kyong Jin Park, Taehyen Kim, Yeon-pyo Hong, Yun-Jung Yang
Environmental Health and Preventive Medicine.2021;[Epub] CrossRef - Urinary Phthalate Levels Associated with the Risk of Nonalcoholic Fatty Liver Disease in Adults: The Korean National Environmental Health Survey (KoNEHS) 2012–2014
Yun-Jung Yang, Taehyen Kim, Yeon-Pyo Hong
International Journal of Environmental Research and Public Health.2021; 18(11): 6035. CrossRef - Benzyl Butyl Phthalate Induced Early lncRNA H19 Regulation in C3H10T1/2 Stem Cell Line
Jian Zhang, Mahua Choudhury
Chemical Research in Toxicology.2021; 34(1): 54. CrossRef - Relationship between urinary phthalate metabolites and diabetes: Korean National Environmental Health Survey (KoNEHS) cycle 3 (2015–2017)
Do Jin Nam, Yeji Kim, Eun Hye Yang, Hyo Choon Lee, Jae-Hong Ryoo
Annals of Occupational and Environmental Medicine.2020;[Epub] CrossRef - Urinary bisphenol A, phthalate metabolites, and obesity: do gender and menopausal status matter?
Jung-eun Lim, BongKyoo Choi, Sun Ha Jee
Environmental Science and Pollution Research.2020; 27(27): 34300. CrossRef - Phthalate metabolites and biomarkers of oxidative stress in the follicular fluid of women undergoing in vitro fertilization
Xiao-Qiong Yuan, Yao-Yao Du, Chong Liu, Na Guo, Xue-Mei Teng, Xiang Hua, Yang-Cheng Yao, Yan-Ling Deng, Qiang Zeng, Tao-Ran Deng, Yu-Feng Li
Science of The Total Environment.2020; 738: 139834. CrossRef
- Figure
- Related articles
-
- Association between outdoor clothing use and serum perfluoroalkyl substances (PFAS): Korean National Environmental Health Survey cycle 4
- Relationship between the use of hair products and urine benzophenone-3: the Korean National Environmental Health Survey (KoNEHS) cycle 4
- Relationship between crustacean consumption and serum perfluoroalkyl substances (PFAS): the Korean National Environmental Health Survey (KoNEHS) cycle 4
- Association between serum perfluoroalkyl substances concentrations and non-alcoholic fatty liver disease among Korean adults: a cross-sectional study using the National Environmental Health Survey cycle 4
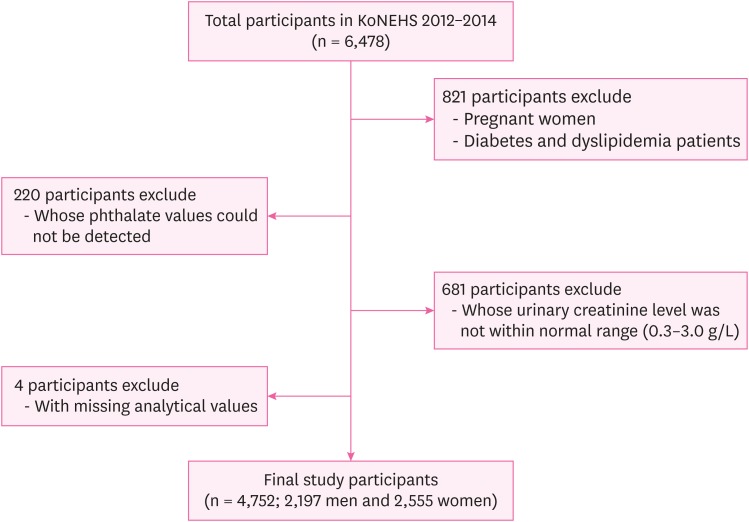
Fig. 1
| Characteristics | Category | Total | Men | Women |
|---|---|---|---|---|
| No. (%*) | No. (%*) | No. (%*) | ||
| Total | - | 4,752 (100) | 2,197 (52.76) | 2,555 (47.24) |
| Age (years) | 19–29 | 457 (19.42) | 229 (19.67) | 228 (19.14) |
| 30–39 | 868 (21.72) | 390 (22.38) | 478 (21.00) | |
| 40–49 | 1,003 (23.12) | 451 (23.71) | 552 (22.45) | |
| 50–59 | 1,051 (18.80) | 454 (18.84) | 597 (18.75) | |
| 60–69 | 858 (9.14) | 418 (9.00) | 440 (9.29) | |
| ≥ 70 | 515 (7.80) | 255 (6.40) | 260 (9.37) | |
| Marital status | Single | 974 (26.69) | 401 (26.05) | 573 (27.41) |
| Married | 3,778 (73.31) | 1,796 (73.95) | 1,982 (72.59) | |
| Household income | 1st quartile | 1,080 (15.61) | 460 (13.72) | 620 (17.72) |
| 2nd quartile | 1,234 (24.06) | 577 (23.55) | 657 (24.62) | |
| 3rd quartile | 1,328 (32.34) | 640 (34.31) | 688 (30.15) | |
| 4th quartile | 1,110 (27.99) | 520 (28.42) | 590 (27.51) | |
| Education | ≤ Middle school | 1,467 (19.77) | 562 (15.49) | 905 (24.55) |
| High school | 1,474 (30.96) | 669 (30.21) | 805 (31.80) | |
| ≥ College | 1,811 (49.27) | 966 (54.30) | 845 (43.65) | |
| Smoking status | Non-smoker† | 3,833 (76.86) | 1,380 (59.78) | 2,453 (95.93) |
| Current-smoker | 919 (23.14) | 817 (40.22) | 102 (4.07) | |
| Exercise | No | 3,472 (73.65) | 1,559 (70.62) | 1,913 (77.04) |
| Yes‡ | 1,280 (26.35) | 638 (29.38) | 642 (22.96) | |
| Drinking | None | 1,509 (27.30) | 341 (7.67) | 1,168 (19.63) |
| Light drinker§ | 1,541 (32.02) | 618 (15.15) | 923 (16.87) | |
| Heavy drinker‖ | 1,702 (40.68) | 1,238 (29.94) | 464 (10.75) | |
| Body mass index | Normal/under weight | 2,952 (63.65) | 1,274 (57.04) | 1,678 (71.03) |
| Overweight | 1,535 (30.42) | 798 (35.99) | 737 (24.19) | |
| Obese | 265 (5.93) | 125 (6.97) | 140 (4.78) |
| Characteristics | Normal/under weight | Overweight | Obese | p-value¶ | |
|---|---|---|---|---|---|
| Category | %* (SE) | ||||
| Age (years) | 19–29 | 73.88 (2.67) | 17.95 (2.26) | 8.17 (1.66) | < 0.0001†† |
| 30–39 | 62.53 (1.90) | 29.46 (1.71) | 8.01 (1.06) | ||
| 40–49 | 63.17 (1.93) | 31.46 (1.87) | 5.37 (0.81) | ||
| 50–59 | 61.23 (1.86) | 34.40 (1.76) | 4.37 (0.80) | ||
| 60–69 | 56.18 (1.91) | 39.75 (1.86) | 4.07 (0.74) | ||
| ≥ 70 | 57.29 (2.71) | 40.46 (2.61) | 2.25 (0.76) | ||
| Gender | Men | 57.04 (1.48) | 35.99 (1.33) | 6.97 (0.77) | < 0.0001†† |
| Women | 71.03 (1.21) | 24.19 (1.08) | 4.78 (0.56) | ||
| Marital status | Single | 69.20 (2.21) | 23.84 (1.85) | 6.96 (1.28) | 0.0005†† |
| Married | 61.63 (1.07) | 32.81 (1.00) | 5.56 (0.46) | ||
| Household income | 1st quartile | 61.14 (1.88) | 34.09 (1.85) | 4.77 (0.83) | 0.1236 |
| 2nd quartile | 61.36 (1.91) | 32.71 (1.80) | 5.93 (0.80) | ||
| 3rd quartile | 63.94 (1.77) | 29.46 (1.69) | 6.60 (0.82) | ||
| 4th quartile | 66.67 (2.00) | 27.50 (1.64) | 5.83 (1.00) | ||
| Education | ≤ Middle school | 58.05 (1.70) | 37.70 (1.59) | 4.25 (0.62) | < 0.0001†† |
| High school | 60.20 (1.60) | 32.70 (1.54) | 7.10 (0.82) | ||
| ≥ College | 68.07 (1.51) | 26.06 (1.27) | 5.87 (0.75) | ||
| Smoking | Non-smoker† | 65.43 (1.06) | 29.24 (0.89) | 5.33 (0.50) | 0.0015** |
| Current-smoker | 57.72 (2.12) | 34.32 (2.13) | 7.96 (1.23) | ||
| Exercise | No | 64.46 (1.16) | 29.78 (1.04) | 5.76 (0.51) | 0.4084 |
| Yes‡ | 61.37 (1.84) | 32.20 (1.66) | 6.43 (1.22) | ||
| Drinking | None | 66.90 (1.54) | 27.02 (1.42) | 6.08 (0.82) | < 0.0006†† |
| Light drinker§ | 66.61 (1.66) | 28.90 (1.49) | 4.49 (0.78) | ||
| Heavy drinker‖ | 59.14 (1.64) | 33.88 (1.53) | 6.98 (0.80) | ||
| Characteristics | Total | Normal/under weight | Overweight | Obese | p-value∥ | ||
|---|---|---|---|---|---|---|---|
| Geometric mean (SE) | |||||||
| Total (n = 4,752) | |||||||
| DEHP | 67.86 (1.04) | 68.16 (1.22)* | 67.56 (1.64)* | 66.21 (3.89)* | 0.0158¶ | ||
| MEHHP | 23.84 (0.41) | 23.66 (0.47)* | 24.14 (0.61)† | 24.25 (1.46)‡ | < 0.0001†† | ||
| MEOHP | 16.45 (0.28) | 16.68 (0.32) | 16.12 (0.43) | 15.73 (1.01) | 0.2109 | ||
| MECPP | 26.60 (0.40) | 26.84 (0.49) | 26.34 (0.64) | 25.40 (1.45) | 0.1369 | ||
| MBzP | 3.69 (0.13) | 3.65 (0.14)* | 3.73 (0.17)* | 3.94 (0.31)* | 0.0118¶ | ||
| MnBP | 32.18 (0.82) | 33.25 (0.90)* | 30.74 (1.02)* | 28.59 (1.65)* | 0.0379¶ | ||
| Men (n = 2,197) | |||||||
| DEHP | 57.96 (1.06) | 59.65 (1.32) | 55.78 (1.68) | 55.80 (4.06) | 0.6256 | ||
| MEHHP | 20.66 (0.42) | 20.99 (0.52) | 20.12 (0.64) | 20.85 (1.58) | 0.6582 | ||
| MEOHP | 13.76 (0.28) | 14.27 (0.35) | 13.13 (0.44) | 13.00 (1.04) | 0.4682 | ||
| MECPP | 22.74 (0.40) | 23.53 (0.50) | 21.83 (0.65) | 21.24 (1.50) | 0.3517 | ||
| MBzP | 3.26 (0.12) | 3.25 (0.14) | 3.21 (0.18) | 3.57 (0.34) | 0.3215 | ||
| MnBP | 27.38 (0.79) | 28.70 (0.98)* | 25.88 (0.96)* | 24.90 (1.88)* | 0.0241¶ | ||
| Women (n = 2,555) | |||||||
| DEHP | 80.92 (1.42) | 76.81 (1.63)* | 92.88 (2.64)† | 87.48 (6.65)§ | < 0.0001†† | ||
| MEHHP | 27.97 (0.54) | 26.34 (0.61)* | 32.69 (0.97)† | 31.02 (2.45)§ | < 0.0001†† | ||
| MEOHP | 20.08 (0.38) | 19.17 (0.43)* | 22.68 (0.68)† | 21.47 (1.75)‡ | < 0.0001†† | ||
| MECPP | 31.69 (0.59) | 30.19 (0.68)* | 35.99 (1.09)† | 34.00 (2.53)§ | < 0.0001†† | ||
| MBzP | 4.24 (0.16) | 4.05 (0.18)* | 4.78 (0.24)† | 4.63 (0.51)‡ | 0.0002†† | ||
| MnBP | 38.54 (0.99) | 37.95 (1.04)* | 40.90 (1.60)* | 35.81 (2.45)* | 0.0075** | ||
| Characteristics | Men (n = 2,197) | Women (n = 2,555) | |||||
|---|---|---|---|---|---|---|---|
| Crude | Model 1* | Model 2† | Crude | Model 1* | Model 2† | ||
| DEHP | |||||||
| Q1 | Ref | Ref | Ref | Ref | Ref | Ref | |
| Q2 | 0.87 (0.67–1.14) | 0.91 (0.69–1.18) | 0.91 (0.69–1.19) | 1.42 (0.98–2.06) | 1.18 (0.81–1.70) | 1.17 (0.80–1.69) | |
| Q3 | 0.91 (0.67–1.23) | 0.98 (0.72–1.34) | 0.98 (0.72–1.35) | 1.80 (1.26–2.59) | 1.22 (0.84–1.77) | 1.22 (0.84–1.77) | |
| Q4 | 0.77 (0.54–1.08) | 0.88 (0.62–1.25) | 0.86 (0.60–1.24) | 2.46 (1.70–3.58) | 1.51 (1.04–2.21) | 1.52 (1.04–2.21) | |
| MEHHP | |||||||
| Q1 | Ref | Ref | Ref | Ref | Ref | Ref | |
| Q2 | 0.92 (0.71–1.20) | 0.93 (0.72–1.21) | 0.94 (0.72–1.22) | 1.56 (1.08–2.24) | 1.27 (0.87–1.84) | 1.28 (0.88–1.85) | |
| Q3 | 0.90 (0.66–1.25) | 0.97 (0.69–1.36) | 0.97 (0.69–1.36) | 1.87 (1.32–2.65) | 1.30 (0.91–1.88) | 1.31 (0.91–1.87) | |
| Q4 | 0.95 (0.68–1.33) | 1.08 (0.77–1.52) | 1.07 (0.75–1.51) | 2.67 (1.86–3.84) | 1.71 (1.18–2.48) | 1.72 (1.19–2.49) | |
| MEOHP | |||||||
| Q1 | Ref | Ref | Ref | Ref | Ref | Ref | |
| Q2 | 0.87 (0.68–1.13) | 0.92 (0.71–1.19) | 0.92 (0.71–1.20) | 1.57 (1.10–2.25) | 1.28 (0.88–1.85) | 1.27 (0.88–1.83) | |
| Q3 | 0.92 (0.66–1.27) | 0.99 (0.70–1.40) | 0.99 (0.70–1.41) | 1.62 (1.15–2.30) | 1.13 (0.79–1.63) | 1.14 (0.79–1.64) | |
| Q4 | 0.78 (0.55–1.10) | 0.89 (0.63–1.26) | 0.89 (0.62–1.27) | 2.22 (1.57–3.13) | 1.33 (0.93–1.91) | 1.33 (0.93–1.90) | |
| MECPP | |||||||
| Q1 | Ref | Ref | Ref | Ref | Ref | Ref | |
| Q2 | 0.84 (0.65–1.09) | 0.87 (0.67–1.13) | 0.87 (0.67–1.13) | 1.26 (0.89–1.77) | 1.06 (0.74–1.53) | 1.05 (0.73–1.50) | |
| Q3 | 0.86 (0.63–1.16) | 0.93 (0.68–1.27) | 0.93 (0.69–1.27) | 1.45 (1.01–2.09) | 1.07 (0.72–1.58) | 1.06 (0.72–1.57) | |
| Q4 | 0.76 (0.53–1.08) | 0.89 (0.61–1.30) | 0.87 (0.60–1.28) | 2.09 (1.47–2.96) | 1.32 (0.91–1.89) | 1.31 (0.91–1.88) | |
| MBzP | |||||||
| Q1 | Ref | Ref | Ref | Ref | Ref | Ref | |
| Q2 | 0.92 (0.69–1.23) | 0.94 (0.70–1.25) | 0.93 (0.70–1.24) | 0.96 (0.69–1.33) | 0.86 (0.62–1.21) | 0.88 (0.63–1.22) | |
| Q3 | 0.99 (0.73–1.33) | 1.06 (0.79–1.42) | 1.03 (0.77–1.39) | 1.36 (0.98–1.88) | 1.12 (0.80–1.58) | 1.15 (0.82–1.61) | |
| Q4 | 1.05 (0.74–1.49) | 1.14 (0.81–1.61) | 1.13 (0.80–1.60) | 1.38 (1.01–1.89) | 1.01 (0.73–1.41) | 1.03 (0.74–1.43) | |
| MnBP | |||||||
| Q1 | Ref | Ref | Ref | Ref | Ref | Ref | |
| Q2 | 0.85 (0.66–1.10) | 0.84 (0.65–1.08) | 0.85 (0.66–1.09) | 0.86 (0.63–1.18) | 0.79 (0.56–1.11) | 0.80 (0.57–1.12) | |
| Q3 | 0.75 (0.55–1.02) | 0.75 (0.56–1.02) | 0.76 (0.56–1.03) | 1.01 (0.74–1.36) | 0.84 (0.60–1.18) | 0.85 (0.61–1.19) | |
| Q4 | 0.66 (0.47–0.92) | 0.70 (0.50–0.97) | 0.71 (0.50–0.99) | 1.12 (0.81–1.55) | 0.82 (0.57–1.16) | 0.83 (0.58–1.18) | |
| Characteristics | Men | Women | |||
|---|---|---|---|---|---|
| < 50 (n = 1,070) | ≥ 50 (n = 1,127) | < 50 (n = 1,258) | ≥ 50 (n = 1,297) | ||
| DEHP | |||||
| Q1 | Ref | Ref | Ref | Ref | |
| Q2 | 1.06 (0.80–1.42) | 1.08 (0.78–1.50) | 1.01 (0.69–1.48) | 1.64 (1.01–2.67) | |
| Q3 | 1.32 (0.94–1.85) | 0.89 (0.63–1.25) | 1.09 (0.74–1.61) | 1.91 (1.21–3.01) | |
| Q4 | 1.26 (0.82–1.91) | 0.87 (0.61–1.26) | 1.30 (0.87–1.94) | 2.04 (1.31–3.18) | |
| MEHHP | |||||
| Q1 | Ref | Ref | Ref | Ref | |
| Q2 | 1.01 (0.75–1.35) | 0.97 (0.69–1.35) | 1.19 (0.81–1.76) | 1.29 (0.82–2.03) | |
| Q3 | 1.37 (0.98–1.91) | 1.06 (0.76–1.50) | 1.08 (0.73–1.59) | 1.76 (1.15–2.71) | |
| Q4 | 1.47 (0.97–2.21) | 0.92 (0.64–1.32) | 1.47 (0.99–2.18) | 1.94 (1.28–2.94) | |
| MEOHP | |||||
| Q1 | Ref | Ref | Ref | Ref | |
| Q2 | 1.27 (0.84–1.50) | 1.01 (0.73–1.40) | 0.93 (0.63–1.38) | 1.96 (1.21–3.18) | |
| Q3 | 1.26 (0.90–1.77) | 0.82 (0.58–1.16) | 1.09 (0.75–1.59) | 1.68 (1.06–2.65) | |
| Q4 | 1.35 (0.87–2.10) | 0.80 (0.56–1.15) | 1.01 (0.67–1.50) | 1.88 (1.21–2.94) | |
| MECPP | |||||
| Q1 | Ref | Ref | Ref | Ref | |
| Q2 | 0.92 (0.69–1.22) | 1.22 (0.87–1.70) | 0.82 (0.56–1.20) | 1.37 (0.87–2.15) | |
| Q3 | 1.37 (0.98–1.92) | 0.96 (0.69–1.34) | 1.00 (0.68–1.47) | 1.18 (0.77–1.83) | |
| Q4 | 1.18 (0.76–1.83) | 0.82 (0.57–1.18) | 1.36 (0.92–1.99) | 1.43 (0.95–2.17) | |
| MBzP | |||||
| Q1 | Ref | Ref | Ref | Ref | |
| Q2 | 0.96 (0.71–1.31) | 0.90 (0.64–1.26) | 0.79 (0.55–1.13) | 1.20 (0.83–1.74) | |
| Q3 | 1.14 (0.81–1.59) | 1.17 (0.84–1.64) | 1.24 (0.86–1.79) | 1.45 (1.02–2.05) | |
| Q4 | 1.36 (0.96–1.92) | 0.94 (0.66–1.32) | 1.00 (0.69–1.47) | 1.27 (0.91–1.78) | |
| MnBP | |||||
| Q1 | Ref | Ref | Ref | Ref | |
| Q2 | 0.83 (0.62–1.11) | 1.21 (0.87–1.69) | 0.75 (0.51–1.09) | 1.14 (0.77–1.68) | |
| Q3 | 0.85 (0.61–1.20) | 0.78 (0.55–1.11) | 0.81 (0.56–1.18) | 1.01 (0.70–1.45) | |
| Q4 | 0.89 (0.60–1.32) | 0.93 (0.69–1.31) | 0.79 (0.54–1.17) | 1.05 (0.75–1.49) | |
*Weighted percentages after accounting for the sampling design; †Non-smoker: never smoked or quit smoking; ‡Exercise: exercising ≥ 3 times a week for ≥ 20 minutes with sweating; §Light drinker: drinking less than heavy drinker; ‖Heavy drinker: drinking ≥ 2 times a week and ≥ 7 glasses for men (5 glasses for women).
SE: standard error.
*Weighted percentages after accounting for the sampling design; †Non-smoker: never smoked or quit smoking; ‡Exercise: exercising ≥ 3 times a week for ≥ 20 minutes with sweating; §Light drinker: drinking less than heavy drinker; ‖Heavy drinker: drinking ≥ 3 times a week and ≥ 7 glasses for men (5 glasses for women); ¶
SE: standard error; DEHP: di-(2-ethyl-5-carboxypentyl) phthalate; MEHHP: mono-(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP: mono-(2-ethyl-5-oxohexyl) phthalate; MECPP: mono-(2-ethyl-5-carboxypentyl) phthalate; MBzP: mono-benzyl phthalate; MnBP: mono-n-butyl phthalate.
*,†,‡,§Turkey multiple comparison test; ‖
DEHP: di-(2-ethyl-5-carboxypentyl) phthalate; MEHHP: mono-(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP: mono-(2-ethyl-5-oxohexyl) phthalate; MECPP: mono-(2-ethyl-5-carboxypentyl) phthalate; MBzP: mono-benzyl phthalate; MnBP: mono-n-butyl phthalate; Ref: reference.
*Adjusted for age, education, marital status, and household income; †Adjusted for age, education, marital status, household income, exercise, smoking, and alcohol consumption.
ORs adjusted for education, marital status, household income, exercise, smoking, and alcohol consumption.
OR, odds ratio; DEHP: di-(2-ethyl-5-carboxypentyl) phthalate; MEHHP: mono-(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP: mono-(2-ethyl-5-oxohexyl) phthalate; MECPP: mono-(2-ethyl-5-carboxypentyl) phthalate; MBzP: mono-benzyl phthalate; MnBP: mono-n-butyl phthalate; Ref: reference.
 KSOEM
KSOEM

 Cite
Cite

