Articles
- Page Path
- HOME > Ann Occup Environ Med > Volume 30; 2018 > Article
- Research Article Environmental exposure of heavy metal (lead and cadmium) and hearing loss: data from the Korea National Health and Nutrition Examination Survey (KNHANES 2010–2013)
-
Gu Hyeok Kang, Jun Young Uhm, Young Gon Choi, Eun Kye Kang, Soo Young Kim, Won Oh Choo, Seong Sil Chang

-
Annals of Occupational and Environmental Medicine 2018;30:22.
DOI: https://doi.org/10.1186/s40557-018-0237-9
Published online: April 17, 2018
Xgrid.411061.3Department of Occupational & Environmental Medicine, Eulji University Hospital, 95 Dunsanseo-ro, Seo-gu, Daejeon, 35233 Republic of Korea
© The Author(s). 2018
Open AccessThis article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Abstract
-
Background Lead and cadmium have been identified as risk factors for hearing loss in animal studies, but large-scale studies targeting the general human population are rare. This study was conducted to investigate the link between heavy metal concentrations in blood and hearing impairment, using a national population-based survey.
-
Methods The study participants comprised 6409 Koreans aged 20 or older, who were included in the Fifth and Sixth Korea National Health and Nutrition Examination Surveys (KNHANES 2010–2013). Hearing impairment was categorized into two types, low- and high-frequency hearing impairment, using pure tone audiometry. Low-frequency hearing impairment was defined as having a binaural average of hearing thresholds for 0.5, 1, and 2 kHz exceeding 25 dB, and high-frequency hearing impairment was defined as having a binaural average of hearing thresholds for 3, 4, and 6 kHz exceeding 25 dB. The blood levels of heavy metals (lead and cadmium) were classified into quartiles. Cross-sectional association between hearing impairment and the level of heavy metals (lead and cadmium) was examined in both sexes. Multivariate logistic regression was used to obtain adjusted odds ratios (ORs) and 95% confidence intervals (CIs).
-
Results Among men, the prevalence of low- and high- frequency hearing impairment was 13.9% and 46.7%, respectively, which was higher than the prevalence among women (11.8% and 27.0%, respectively). Regarding lead, the adjusted OR of high-frequency hearing impairment for the highest blood level group versus the lowest group was significant in both men (OR = 1.629, 95% CI = 1.161–2.287) and women (OR = 1.502, 95% CI = 1.027–2.196), after adjusting for age, body mass index, education, smoking, alcohol consumption, exercise, diagnosis of diabetes mellitus, hypertension, and noise exposure (occupational, loud, firearm noises). No links were found between blood lead levels and low-frequency hearing impairment, or between blood cadmium levels and low- or high-frequency hearing impairment in either sex.
-
Conclusions The present study findings suggest that even exposure to low-level lead is a risk factor for high-frequency hearing loss. A prospective epidemiologic study should be conducted to identify the causal relationship between human health and exposure to heavy metals, and efforts to reduce heavy metal exposure in the general population should continue.
Background
Methods
Results
Discussion
Conclusion
Availability of data and materials
Abbreviations
BMI
CI
KNHANES
OR
OSHA
WHO
- 1. Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and nutrition examination survey, 1999-2004. Arch Intern Med 2008;168(14):1522–1530. 10.1001/archinte.168.14.1522. 18663164.ArticlePubMed
- 2. Cruickshanks KJ, Wiley TL, Tweed TS, Klein BE, Klein R, Mares-Perlman JA, Nondahl DM. Prevalence of hearing loss in older adults in beaver dam, Wisconsin the epidemiology of hearing loss study. Am J Epidemiol 1998;148(9):879–886. 10.1093/oxfordjournals.aje.a009713. 9801018.ArticlePubMed
- 3. http://www.who.int/mediacentre/factsheets/fs300/en/.
- 4. Hong JW, Jeon JH, Ku CR, Noh JH, Yoo HJ, Kim D-J. The prevalence and factors associated with hearing impairment in the Korean adults: the 2010–2012 Korea National Health and nutrition examination survey (observational study). Medicine 2015;94(10):e611. 10.1097/MD.0000000000000611. 25761183.PubMedPMC
- 5. Brink P, Stones M. Examination of the relationship among hearing impairment, linguistic communication, mood, and social engagement of residents in complex continuing-care facilities. The Gerontologist 2007;47(5):633–641. 10.1093/geront/47.5.633. 17989405.ArticlePubMedPDF
- 6. Chia E-M, Wang JJ, Rochtchina E, Cumming RR, Newall P, Mitchell P. Hearing impairment and health-related quality of life: the Blue Mountains hearing study. Ear Hear 2007;28(2):187–195. 10.1097/AUD.0b013e31803126b6. 17496670.ArticlePubMed
- 7.
- 8. Choi Y-H, Hu H, Tak S, Mukherjee B, Park SK. Occupational noise exposure assessment using O* NET and its application to a study of hearing loss in the US general population. Occup Environ Med 2012;69(3):176–183. 10.1136/oem.2011.064758. 21725070.ArticlePubMedPMC
- 9. Vyskocil A, Truchon G, Leroux T, Lemay F, Gendron M, Gagnon F, Majidi NE, Boudjerida A, Lim S, Emond CA. Weight of evidence approach for the assessment of the ototoxic potential of industrial chemicals. Toxicol Ind Health 2012;28(9):796–819. 10.1177/0748233711425067. 22064681.ArticlePubMedPDF
- 10.
- 11. Fuente A, Slade MD, Taylor T, Morata TC, Keith RW, Sparer J, Rabinowitz PM. Peripheral and central auditory dysfunction induced by occupational exposure to organic solvents. J Occup Environ Med 2009;51(10):1202–1211. 10.1097/JOM.0b013e3181bae17c. 19786896.ArticlePubMed
- 12. Jones LG, Prins J, Park S, Walton JP, Luebke AE, Lurie DI. Lead exposure during development results in increased neurofilament phosphorylation, neuritic beading, and temporal processing deficits within the murine auditory brainstem. J Comp Neurol 2008;506(6):1003–1017. 10.1002/cne.21563. 18085597.ArticlePubMed
- 13. Ozcaglar HU, Agirdir B, Dinc O, Turhan M, Kilinçarslan S, Oner G. Effects of cadmium on the hearing system. Acta Otolaryngol 2001;121(3):393–397. 10.1080/000164801300102897. 11425207.ArticlePubMed
- 14. Kim S-J, Jeong H-J, Myung N-Y, Kim M-c, Lee J-H, So H-s, Park R-K, Kim H-M, Um J-Y, Hong S-H. The protective mechanism of antioxidants in cadmium-induced ototoxicity in vitro and in vivo. Environ Health Perspect 2008;116(7):854–862. 10.1289/ehp.10467. 18629305.ArticlePubMedPMC
- 15. Prasher D. Heavy metals and noise exposure: health effects. Noise Health 2009;11(44):141–144. 10.4103/1463-1741.53358. 19602766.ArticlePubMed
- 16. Park SK, Elmarsafawy S, Mukherjee B, Spiro A, Vokonas PS, Nie H, Weisskopf MG, Schwartz J, Hu H. Cumulative lead exposure and age-related hearing loss: the VA normative aging study. Hear Res 2010;269(1):48–55. 10.1016/j.heares.2010.07.004. 20638461.ArticlePubMedPMC
- 17. Shargorodsky J, Curhan SG, Henderson E, Eavey R, Curhan GC. Heavy metals exposure and hearing loss in US adolescents. Arch Otolaryngol Head Neck Surg 2011;137(12):1183–1189. 10.1001/archoto.2011.202. 22183895.ArticlePubMed
- 18. Choi Y-H, Hu H, Mukherjee B, Miller J, Park SK. Environmental cadmium and lead exposures and hearing loss in US adults: the National Health and nutrition examination survey, 1999 to 2004. Environ Health Perspect 2012;120(11):1544. 10.1289/ehp.1104863. 22851306.ArticlePubMedPMC
- 19.
- 20. Rosin A. The long-term consequences of exposure to lead. Isr Med Assoc J 2009;11(11):689–694. 20108558.PubMed
- 21. Henson MC, Chedrese PJ. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp Biol Med 2004;229(5):383–392. 10.1177/153537020422900506.ArticlePDF
- 22. Lee B-K. The role of biological monitoring in the health management of lead-exposed workers. Toxicol Lett 1999;108(2):149–160. 10.1016/S0378-4274(99)00083-1. 10511256.ArticlePubMed
- 23. Mercier M. International approach of the assessment of chemical risks. J Hyg Epidemiol Microbiol Immunol 1990;34(1):1–7. 2351814.PubMed
- 24. Seo J-W, Kim B-G, Kim Y-M, Kim R-B, Chung J-Y, Lee K-M, Hong Y-S. Trend of blood lead, mercury, and cadmium levels in Korean population: data analysis of the Korea National Health and nutrition examination survey. Environ Monit Assess 2015;187(3):146. 10.1007/s10661-015-4348-2. 25716526.ArticlePubMedPDF
- 25. http://www.who.int/pbd/deafness/hearing_impairment_grades/en.
- 26. Picard M, Girard SA, Simard M, Larocque R, Leroux T, Turcotte F. Association of work-related accidents with noise exposure in the workplace and noise-induced hearing loss based on the experience of some 240,000 person-years of observation. Accid Anal Prev 2008;40(5):1644–1652. 10.1016/j.aap.2008.05.013. 18760091.ArticlePubMed
- 27. McBride D, Williams S. Audiometric notch as a sign of noise induced hearing loss. Occup Environ Med 2001;58(1):46–51. 10.1136/oem.58.1.46. 11119634.ArticlePubMedPMC
- 28. Hwang JH, Wu CC, Hsu CJ, Liu TC, Yang WS. Association of central obesity with the severity and audiometric configurations of age-related hearing impairment. Obesity 2009;17(9):1796–1801. 10.1038/oby.2009.66. 19300432.ArticlePubMedPDF
- 29. Itoh A, Nakashima T, Arao H, Wakai K, Tamakoshi A, Kawamura T, Ohno Y. Smoking and drinking habits as risk factors for hearing loss in the elderly: epidemiological study of subjects undergoing routine health checks in Aichi, Japan. Public Health 2001;115(3):192–196. 11429714.ArticlePubMed
- 30. Trune DR, Nguyen-Huynh A. Vascular pathophysiology in hearing disorders. Semin Hear 2012;33(3):242–250. 10.1055/s-0032-1315723. 25346568.ArticlePubMedPMC
- 31. Cristell M, Hutchinson KM, Alessio HM. Effects of exercise training on hearing ability. Scand Audiol 1998;27(4):219–224. 10.1080/010503998420522. 9832404.ArticlePubMed
- 32. Fransen E, Topsakal V, Hendrickx J-J, Van Laer L, Huyghe JR, Van Eyken E, Lemkens N, Hannula S, Mäki-Torkko E, Jensen M. Occupational noise, smoking, and a high body mass index are risk factors for age-related hearing impairment and moderate alcohol consumption is protective: a European population-based multicenter study. J Assoc Res Otolaryngol 2008;9(3):264–276. 10.1007/s10162-008-0123-1. 18543032.ArticlePubMedPMCPDF
- 33. Hirata M, Kosaka H. Effects of lead exposure on neurophysiological parameters. Environ Res 1993;63(1):60–69. 10.1006/enrs.1993.1127. 8404776.ArticlePubMed
- 34. Sabolić I. Common mechanisms in nephropathy induced by toxic metals. Nephron Physiol 2006;104(3):107–114. 10.1159/000095539.
- 35.
- 36. Guallar E, Silbergeld EK, Navas-Acien A, Malhotra S, Astor BC, Sharrett AR, Schwartz BS. Confounding of the relation between homocysteine and peripheral arterial disease by lead, cadmium, and renal function. Am J Epidemiol 2006;163(8):700–708. 10.1093/aje/kwj090. 16484446.ArticlePubMed
- 37. Navas-Acien A, Tellez-Plaza M, Guallar E, Muntner P, Silbergeld E, Jaar B, Weaver V. Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol 2009;170(9):1156–1164. 10.1093/aje/kwp248. 19700501.ArticlePubMedPMC
- 38.
REFERENCES
Notes
Figure & Data
REFERENCES
Citations

- Machine learning-based prediction of hearing loss: Findings of the US NHANES from 2003 to 2018
Yi Mi, Pin Sun
Hearing Research.2025; 461: 109252. CrossRef - Industrial air pollution and newborn hearing screening failure
Yanhong Huang, Yan Lin, Roberta P. Lavin, Li Luo, Ming Luo, Shuguang Leng, Netanya M. Mullen, Karen Hawley, Xi Gong
Journal of Hazardous Materials.2025; 492: 138241. CrossRef - Occupational Lead Exposure Ototoxicity Evaluated With Distortion-Product Otoacoustic Emissions
Soledad Solis-Angeles, Luz María Del Razo, Guadalupe Aguilar-Madrid, Carmina Jiménez-Ramírez, Laura Coco, Alejandro Cabello-López, Cuauhtémoc Arturo Juárez-Pérez
Ear & Hearing.2024; 45(2): 329. CrossRef - Higher exposure to 1,3-butadiene is associated with more severe hearing loss
Sang-Yoon Han, Sang-Yeon Lee, Myung-Whan Suh, Jun Ho Lee, Moo Kyun Park
Scientific Reports.2024;[Epub] CrossRef - Crafting Strategies for Promoting Healthy Ear and Hearing Care: Making It Happen
Saurabh Ram Shrivastava, Prateek Sudhakar Bobhate, Harshal Gajanan Mendhe, Gulshan R Bandre
Noise and Health.2024; 26(122): 354. CrossRef - Hearing Loss and Disorders: The Repercussions of Climate Change
Sue Sherratt
American Journal of Audiology.2023; 32(4): 793. CrossRef - The role of calcium, Akt and ERK signaling in cadmium-induced hair cell death
Jennifer Galdieri, Chloe Adams, María Padilla, Tamara M. Stawicki
Molecular and Cellular Neuroscience.2023; 124: 103815. CrossRef - Combined effects of multiple metals on hearing loss: A Bayesian kernel machine regression approach
Mingming Liang, Xianwei Guo, Xiuxiu Ding, Qiuxia Song, Hao Wang, Ning Li, Wanying Su, Qiwei Liang, Yehuan Sun
Ecotoxicology and Environmental Safety.2022; 247: 114279. CrossRef - Metformin attenuates cadmium-induced degeneration of spiral ganglion neuron via restoring autophagic flux in primary culture
Qian Li, Liuqian Wang, Di Ji, Wei Yu, Yan Zhang, Yanghong Xiang, Chao Zhou, Liting Wang, Ping Deng, Huifeng Pi, Yonghui Lu, Qinlong Ma, Mindi He, Lei Zhang, Zhengping Yu, Anchun Deng
Journal of Inorganic Biochemistry.2022; 234: 111901. CrossRef - Effects of cadmium and high-fat diet on essential metal concentration in the mouse testis
Bin Zhou, Adrienne Gentry, Qian Xu, Jamie L. Young, Xiaofang Yan, Kelly Pagidas, Yu Yang, Walter H. Watson, Maiying Kong, Lu Cai, Jonathan H. Freedman
Toxicology Reports.2021; 8: 718. CrossRef - Metal Exposures, Noise Exposures, and Audiometry from E-Waste Workers in Agbogbloshie, Ghana
Krystin Carlson, Niladri Basu, Julius N. Fobil, Richard L. Neitzel
International Journal of Environmental Research and Public Health.2021; 18(18): 9639. CrossRef - Mechanotransduction Activity Facilitates Hair Cell Toxicity Caused by the Heavy Metal Cadmium
Caleigh Schmid, Isabella Alampi, Jay Briggs, Kelly Tarcza, Tamara M. Stawicki
Frontiers in Cellular Neuroscience.2020;[Epub] CrossRef - Disruption of essential metal homeostasis in the brain by cadmium and high-fat diet
John C. Mazzocco, Rekha Jagadapillai, Evelyne Gozal, Maiying Kong, Qian Xu, Gregory N. Barnes, Jonathan H. Freedman
Toxicology Reports.2020; 7: 1164. CrossRef - Exposure to lead, mercury, styrene, and toluene and hearing impairment: evaluation of dose-response relationships, regulations, and controls
Ehsan Hemmativaghef
Journal of Occupational and Environmental Hygiene.2020; 17(11-12): 574. CrossRef - Association of Blood Cadmium with Cardiovascular Disease in Korea: From the Korea National Health and Nutrition Examination Survey 2008–2013 and 2016
Jihyun Jeong, Sang-moon Yun, Minkyeong Kim, Young Ho Koh
International Journal of Environmental Research and Public Health.2020; 17(17): 6288. CrossRef - Environmental ototoxicants, a potential new class of chemical stressors
Lucia Fábelová, Christopher A. Loffredo, Jana Klánová, Klára Hilscherová, Milena Horvat, Juraj Tihányi, Denisa Richterová, Ľubica Palkovičová Murínová, Soňa Wimmerová, Renata Sisto, Arturo Moleti, Tomáš Trnovec
Environmental Research.2019; 171: 378. CrossRef - Association between cadmium exposure and hearing impairment: a population-based study in Korean adults
Da Jung Jung
Yeungnam University Journal of Medicine.2019; 36(2): 141. CrossRef
- Figure
- Related articles
-
- Relationship between long-term PM2.5 exposure and myopia prevalence in adults: analysis of the Korea National Health and Nutrition Examination Survey–Air Pollution Linked Data, 2020
- Exploring the impact of age and socioeconomic factors on health-related unemployment using propensity score matching: results from Korea National Health and Nutrition Examination Survey (2015–2017)
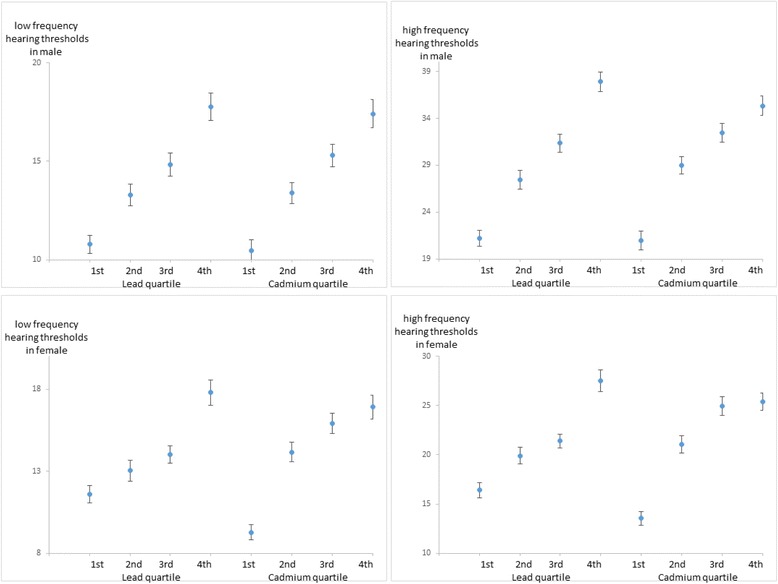
Fig. 1
| Variables | Total na | Male | Female | p-valueb |
|---|---|---|---|---|
| Total | 6409 | 3185 | 3224 | |
| Age (years) | ||||
| Mean ± SE | 47.1 ± 0.3 | 46.3 ± 0.3 | 48.0 ± 0.3 | < 0.001 |
| 20–29 | 1025 | 493 | 532 | 0.002 |
| 30–39 | 1081 | 537 | 544 | |
| 40–49 | 1415 | 709 | 706 | |
| 50–59 | 1454 | 717 | 737 | |
| 60–69 | 1232 | 615 | 617 | |
| 70–87 | 202 | 114 | 88 | |
| BMI (kg/m2) | ||||
| Mean ± SE | 23.8 ± 0.1 | 24.2 ± 0.1 | 23.5 ± 0.1 | < 0.001 |
| Underweight | 246 | 68 | 178 | < 0.001 |
| Normal | 4071 | 1936 | 2135 | |
| Obese | 2092 | 1181 | 911 | |
| Education | ||||
| ≤ Middle school | 1861 | 751 | 1110 | < 0.001 |
| High school | 2365 | 1226 | 1139 | |
| ≥ College | 2183 | 1208 | 975 | |
| Smoking | ||||
| Non-smoker | 3650 | 723 | 2927 | < 0.001 |
| Ex-smoker | 1176 | 1063 | 113 | |
| Current smoker | 1583 | 1399 | 184 | |
| Alcohol | ||||
| None | 638 | 104 | 534 | < 0.001 |
| Light drinker | 4128 | 1923 | 2205 | |
| Heavy drinker | 1643 | 1158 | 485 | |
| Exercise | ||||
| No | 4771 | 2271 | 2500 | < 0.001 |
| Yes | 1638 | 914 | 724 | |
| Current diagnosis of diabetes mellitus | ||||
| No | 5980 | 2936 | 3044 | 0.069 |
| Yes | 429 | 249 | 180 | |
| Current diagnosis of hypertension | ||||
| No | 5237 | 2573 | 2664 | 0.202 |
| Yes | 1172 | 612 | 560 | |
| Occupational noise exposure | ||||
| No | 5496 | 2538 | 2958 | < 0.001 |
| Yes | 913 | 647 | 266 | |
| Loud noise exposure | ||||
| No | 6267 | 3095 | 3172 | 0.008 |
| Yes | 142 | 90 | 52 | |
| Firearm noise exposure | ||||
| No | 4750 | 1642 | 3108 | < 0.001 |
| Yes | 1659 | 1543 | 116 | |
| Variables | Low frequency hearing impairment | High frequency hearing impairment | ||||||
|---|---|---|---|---|---|---|---|---|
| Normala | Impaireda | Rateb | p-valuec | Normala | Impaireda | Rateb | p -valuec | |
| Total | 2743 | 442 | 13.9 | 1697 | 1488 | 46.7 | ||
| Age | ||||||||
| 20–29 | 486 | 7 | 1.4 | < 0.001 | 466 | 27 | 5.5 | < 0.001 |
| 30–39 | 525 | 12 | 2.2 | 455 | 82 | 15.3 | ||
| 40–49 | 680 | 29 | 4.1 | 444 | 265 | 37.4 | ||
| 50–59 | 601 | 116 | 16.2 | 244 | 473 | 66 | ||
| 60–69 | 406 | 209 | 34 | 84 | 531 | 86.3 | ||
| 70–87 | 45 | 69 | 60.5 | 4 | 110 | 96.5 | ||
| BMI (kg/m2) | ||||||||
| Underweight | 56 | 12 | 17.6 | 0.086 | 39 | 29 | 42.6 | 0.610 |
| Normal | 1650 | 286 | 14.8 | 1020 | 916 | 47.3 | ||
| Obese | 1037 | 144 | 12.2 | 638 | 543 | 46 | ||
| Education | ||||||||
| ≤ Middle school | 514 | 237 | 31.6 | < 0.001 | 159 | 592 | 78.8 | < 0.001 |
| High school | 1086 | 140 | 11.4 | 691 | 535 | 43.6 | ||
| ≥ College | 1143 | 65 | 5.4 | 847 | 361 | 29.9 | ||
| Smoking | ||||||||
| Non-smoker | 664 | 59 | 8.2 | < 0.001 | 483 | 240 | 33.2 | < 0.001 |
| Ex-smoker | 860 | 203 | 19.1 | 426 | 637 | 59.9 | ||
| Current smoker | 1219 | 180 | 12.9 | 788 | 611 | 43.7 | ||
| Alcohol | ||||||||
| None | 77 | 27 | 26 | < 0.001 | 42 | 62 | 59.6 | < 0.001 |
| Light drinker | 1613 | 310 | 16.1 | 914 | 1009 | 52.5 | ||
| Heavy drinker | 1053 | 105 | 9.1 | 741 | 417 | 36 | ||
| Exercise | ||||||||
| No | 1941 | 330 | 14.5 | 0.093 | 1195 | 1076 | 47.4 | 0.239 |
| Yes | 802 | 112 | 12.3 | 502 | 412 | 45.1 | ||
| Current diagnosis of diabetes mellitus | ||||||||
| No | 2558 | 378 | 12.9 | < 0.001 | 1639 | 1297 | 44.2 | < 0.001 |
| Yes | 185 | 64 | 25.7 | 58 | 191 | 76.7 | ||
| Current diagnosis of hypertension | ||||||||
| No | 2289 | 284 | 11 | < 0.001 | 1528 | 1045 | 40.6 | < 0.001 |
| Yes | 454 | 158 | 25.8 | 169 | 443 | 72.4 | ||
| Occupational noise exposure | ||||||||
| no | 2222 | 316 | 12.5 | < 0.001 | 1413 | 1125 | 44.3 | < 0.001 |
| yes | 521 | 126 | 19.5 | 284 | 363 | 56.1 | ||
| Loud noise exposure | ||||||||
| no | 2667 | 428 | 13.8 | 0.640 | 1645 | 1450 | 46.8 | 0.386 |
| yes | 76 | 14 | 15.6 | 52 | 38 | 42.2 | ||
| Firearm noise exposure | ||||||||
| no | 1425 | 217 | 13.2 | 0.265 | 884 | 758 | 46.2 | 0.517 |
| yes | 1318 | 225 | 14.6 | 813 | 730 | 47.3 | ||
| Variables | Low frequency hearing impairment | High frequency hearing impairment | ||||||
|---|---|---|---|---|---|---|---|---|
| Normala | Impaireda | Rateb | p-valuec | Normala | Impaireda | Rateb | p-valuec | |
| Total | 2845 | 379 | 11.8 | 2354 | 870 | 27.0 | ||
| Age | ||||||||
| 20–29 | 525 | 7 | 1.3 | < 0.001 | 519 | 13 | 2.4 | < 0.001 |
| 30–39 | 535 | 9 | 1.7 | 522 | 22 | 4 | ||
| 40–49 | 676 | 30 | 4.2 | 614 | 92 | 13 | ||
| 50–59 | 641 | 96 | 13 | 479 | 258 | 35 | ||
| 60–69 | 421 | 196 | 31.8 | 209 | 408 | 66.1 | ||
| 70–87 | 47 | 41 | 46.6 | 11 | 77 | 87.5 | ||
| BMI (kg/m2) | ||||||||
| Underweight | 167 | 11 | 6.2 | < 0.001 | 156 | 22 | 12.4 | < 0.001 |
| Normal | 1906 | 229 | 10.7 | 1603 | 532 | 24.9 | ||
| Obese | 772 | 139 | 15.3 | 595 | 316 | 34.7 | ||
| Education | ||||||||
| ≤ Middle school | 836 | 274 | 24.7 | 0.031 | 510 | 600 | 54.1 | < 0.001 |
| High school | 1058 | 81 | 7.1 | 938 | 201 | 17.6 | ||
| ≥ College | 951 | 24 | 2.5 | 906 | 69 | 7.1 | ||
| Smoking | ||||||||
| Non-smoker | 2569 | 358 | 12.2 | 0.062 | 2114 | 813 | 27.8 | 0.004 |
| Ex-smoker | 105 | 8 | 7.1 | 88 | 25 | 22.1 | ||
| Current smoker | 171 | 13 | 7.1 | 152 | 32 | 17.4 | ||
| Alcohol | ||||||||
| None | 412 | 122 | 22.8 | < 0.001 | 281 | 253 | 47.4 | < 0.001 |
| Light drinker | 1962 | 243 | 11 | 1646 | 559 | 25.4 | ||
| Heavy drinker | 471 | 14 | 2.9 | 427 | 58 | 12 | ||
| Exercise | ||||||||
| No | 2185 | 315 | 12.6 | 0.006 | 1794 | 706 | 28.2 | 0.003 |
| Yes | 660 | 64 | 8.8 | 560 | 164 | 22.7 | ||
| Current diagnosis of diabetes mellitus | ||||||||
| No | 2717 | 327 | 10.7 | < 0.001 | 2272 | 772 | 25.4 | < 0.001 |
| Yes | 128 | 52 | 28.9 | 82 | 98 | 54.4 | ||
| Current diagnosis of hypertension | ||||||||
| No | 2444 | 220 | 8.3 | < 0.001 | 2112 | 552 | 20.7 | < 0.001 |
| Yes | 401 | 159 | 28.4 | 242 | 318 | 56.8 | ||
| Occupational noise exposure | ||||||||
| No | 2610 | 348 | 11.8 | 0.957 | 2174 | 784 | 26.5 | 0.043 |
| Yes | 235 | 31 | 11.7 | 180 | 86 | 32.3 | ||
| Loud noise exposure | ||||||||
| No | 2797 | 375 | 11.8 | 0.359 | 2318 | 854 | 26.9 | 0.535 |
| Yes | 48 | 4 | 7.7 | 36 | 16 | 30.8 | ||
| Firearm noise exposure | ||||||||
| no | 2747 | 361 | 11.6 | 0.200 | 2288 | 820 | 26.4 | < 0.001 |
| yes | 98 | 18 | 15.5 | 66 | 50 | 43.1 | ||
| Analyte | <25th | 25th to <50th | 50th to <75th | ≥75th |
|---|---|---|---|---|
| Lead | ||||
| Conc, μg/dLa | 1.56 ± 0.01 | 2.22 ± 0.01 | 2.82 ± 0.01 | 4.22 ± 0.08 |
| Case/n (low frequency) | 80/796 | 96/797 | 111/796 | 155/796 |
| Hearing thresholds(dB)b | 10.8 ± 0.47 | 13.3 ± 0.56 | 14.8 ± 0.59 | 17.8 ± 0.70 |
| Prevalence(%)c | 9.2% | 12.7% | 16.2% | 21.7% |
| Adjusted OR(95% CI)d | Referent | 1.153 (0.761–1.747) | 1.002 (0.644–1.559) | 1.049 (0.694–1.585) |
| Adjusted OR(95% CI)e | Referent | 1.15 (0.758–1.745) | 0.998 (0.640–1.555) | 1.045 (0.690–1.582) |
| Adjusted OR(95% CI)f | Referent | 1.17 (0.772–1.772) | 1.028 (0.661–1.597) | 1.026 (0.677–1.556) |
| Case/n (high frequency) | 242/796 | 322/797 | 404/796 | 520/796 |
| Hearing thresholds(dB)b | 21.2 ± 0.86 | 27.4 ± 0.99 | 31.4 ± 0.99 | 37.9 ± 1.05 |
| Prevalence(%)c | 28.6% | 41.6% | 51.3% | 63.9% |
| Adjusted OR(95% CI)d | Referent | 1.342 (0.988–1.823) | 1.357 (0.972–1.894) | 1.598 (1.14–2.238) |
| Adjusted OR(95% CI)e | Referent | 1.352 (0.994–1.837) | 1.365 (0.978–1.906) | 1.614 (1.151–2.263) |
| Adjusted OR(95% CI)f | Referent | 1.368 (1.006–1.859) | 1.402 (1.005–1.955) | 1.629 (1.161–2.287) |
| Cadmium | ||||
| Conc, μg/dLa | 0.47 ± 0.01 | 0.79 ± 0.01 | 1.13 ± 0.01 | 1.88 ± 0.03 |
| Case/n (low frequency) | 57/797 | 99/797 | 130/798 | 156/793 |
| Hearing thresholds(dB)b | 10.5 ± 0.55 | 13.4 ± 0.53 | 15.3 ± 0.57 | 17.4 ± 0.71 |
| Prevalence(%)c | 8.4% | 12.9% | 17.1% | 21.1% |
| Adjusted OR(95% CI)d | Referent | 0.857 (0.532–1.379) | 0.845 (0.54–1.322) | 0.924 (0.567–1.505) |
| Adjusted OR(95% CI)e | Referent | 0.855 (0.531–1.376) | 0.84 (0.537–1.313) | 0.922 (0.566–1.503) |
| Adjusted OR(95% CI)f | Referent | 0.842 (0.524–1.354) | 0.83 (0.533–1.291) | 0.905 (0.556–1.473) |
| Case/n (high frequency) | 221/797 | 362/797 | 433/798 | 472/793 |
| Hearing thresholds(dB)b | 20.9 ± 1.00 | 29.0 ± 0.94 | 32.5 ± 1.02 | 35.3 ± 1.04 |
| Prevalence(%)c | 27.4% | 46.3% | 52.3% | 59.2% |
| Adjusted OR(95% CI)d | Referent | 1.238 (0.895–1.712) | 1.159 (0.828–1.623) | 1.284 (0.892–1.848) |
| Adjusted OR(95% CI)e | Referent | 1.236 (0.894–1.71) | 1.163 (0.83–1.629) | 1.283 (0.891–1.848) |
| Adjusted OR(95% CI)f | Referent | 1.235 (0.891–1.711) | 1.168 (0.833–1.638) | 1.292 (0.896–1.865) |
| Analyte | <25th | 25th to <50th | 50th to <75th | ≥75th |
|---|---|---|---|---|
| Lead | ||||
| Conc, μg/dLa | 1.12 ± 0.01 | 1.61 ± 0.01 | 2.11 ± 0.01 | 3.03 ± 0.03 |
| Case/n (low frequency) | 63//808 | 82/804 | 101/806 | 133/806 |
| Hearing thresholds(dB)b | 11.6 ± 0.53 | 13.0 ± 0.63 | 14.0 ± 0.52 | 17.8 ± 0.79 |
| Prevalence(%)c | 10.9% | 11.1% | 12.6% | 20.6% |
| Adjusted OR(95% CI)d | Referent | 1.312 (0.755–2.279) | 1.302 (0.791–2.143) | 0.957 (0.563–1.626) |
| Adjusted OR(95% CI)e | Referent | 1.288 (0.74–2.24) | 1.281 (0.773–2.123) | 0.93 (0.544–1.589) |
| Adjusted OR(95% CI)f | Referent | 1.271 (0.726–2.223) | 1.308 (0.784–2.183) | 0.932 (0.541–1.604) |
| Case/n (high frequency) | 135/808 | 178/804 | 234/806 | 323/806 |
| Hearing thresholds(dB)b | 16.4 ± 0.75 | 19.9 ± 0.87 | 21.4 ± 0.70 | 27.5 ± 1.10 |
| Prevalence(%)c | 20.3% | 24.2% | 29.2% | 44.7% |
| Adjusted OR(95% CI)d | Referent | 0.937 (0.599–1.464) | 1.009 (0.695–1.464) | 1.488 (1.02–2.172) |
| Adjusted OR(95% CI)e | Referent | 0.941 (0.602–1.471) | 1.012 (0.698–1.467) | 1.499 (1.028–2.187) |
| Adjusted OR(95% CI)f | Referent | 0.947 (0.606–1.477) | 1.013 (0.698–1.471) | 1.502 (1.027–2.196) |
| Cadmium | ||||
| Conc, μg/dLa | 0.57 ± 0.01 | 0.96 ± 0.01 | 1.36 ± 0.01 | 2.17 ± 0.03 |
| Case/n (low frequency) | 42/806 | 89/807 | 110/806 | 138/805 |
| Hearing thresholds(dB)b | 9.3 ± 0.45 | 14.2 ± 0.59 | 15.9 ± 0.63 | 16.9 ± 0.73 |
| Prevalence(%)c | 6.2% | 13.4% | 17% | 18.5% |
| Adjusted OR(95% CI)d | Referent | 0.873 (0.486–1.567) | 0.916 (0.523–1.603) | 0.768 (0.432–1.364) |
| Adjusted OR(95% CI)e | Referent | 0.88 (0.489–1.586) | 0.92 (0.524–1.616) | 0.758 (0.426–1.349) |
| Adjusted OR(95% CI)f | Referent | 0.875 (0.488–1.57) | 0.913 (0.521–1.601) | 0.757 (0.427–1.343) |
| Case/n (high frequency) | 85/806 | 212/807 | 280/806 | 293/805 |
| Hearing thresholds(dB)b | 13.5 ± 0.69 | 21.1 ± 0.88 | 25.0 ± 0.94 | 25.4 ± 0.90 |
| Prevalence(%)c | 13.3% | 28.4% | 37.6% | 38.6% |
| Adjusted OR(95% CI)d | Referent | 1.225 (0.799–1.878) | 1.302 (0.821–2.067) | 1.425 (0.91–2.231) |
| Adjusted OR(95% CI)e | Referent | 1.229 (0.799–1.89) | 1.308 (0.821–2.082) | 1.427 (0.908–2.244) |
| Adjusted OR(95% CI)f | Referent | 1.248 (0.812–1.919) | 1.325 (0.831–2.115) | 1.426 (0.906–2.244) |
SE standard error
aunweighted count, btested by chi-square test
aunweighted count
bprevalence rate
ctested by chi-square test
aunweighted count
bprevalence rate
ctested by chi-square test
aConc, weighted mean ± SE
bBinaural average of hearing thresholds, weighted mean ± SE
cWeighted percentages
dAdjusted for age, BMI, education, smoking, alcohol consumption, and exercise,
eAdditional adjustment for diabetes mellitus, hypertension
fAdditional adjustment for noise exposure (Occupational, loud, Firearm noise)
aConc, weighted mean ± SE
bBinaural average of hearing thresholds, weighted mean ± SE
cWeighted percentages
dAdjusted for age, BMI, education, smoking, alcohol consumption, and exercise,
eAdditional adjustment for diabetes mellitus, hypertension
fAdditional adjustment for noise exposure (Occupational, loud, Firearm noise)
 KSOEM
KSOEM

 Cite
Cite

