Articles
- Page Path
- HOME > Ann Occup Environ Med > Volume 35; 2023 > Article
- Original Article Environment-wide association study of elevated liver enzymes: results from the Korean National Environmental Health Survey 2018–2022
-
Youngchan Chi1
 , Jong-Tae Park1
, Jong-Tae Park1 , Sewhan Na1,2
, Sewhan Na1,2 , Kyeongmin Kwak1
, Kyeongmin Kwak1
-
Annals of Occupational and Environmental Medicine 2023;35:e27.
DOI: https://doi.org/10.35371/aoem.2023.35.e27
Published online: July 31, 2023
1Department of Occupational and Environmental Medicine, Korea University Ansan Hospital, Ansan, Korea.
2Department of Environmental Health Sciences, Seoul National University Graduate School of Public Health, Seoul, Korea.
- Correspondence: Kyeongmin Kwak. Department of Occupational and Environmental Medicine, Korea University Ansan Hospital, 123 Jeokgeum-ro, Danwon-gu, Ansan 15355, Korea. pathfinder81@korea.ac.kr
Copyright © 2023 Korean Society of Occupational & Environmental Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background Environmental exposure is characterized by low concentration, chronic, and complex exposure. Traditional epidemiological studies show limitations in reflecting these characteristics since they usually focus on a single or very limited number of exposure factors at a time. In this study, we adopted the methodology of environment-wide association study (EWAS) to figure out the association of human liver function with various environmentally hazardous substances.
-
Methods We analyzed 2,961 participants from the Korean National Environmental Health Survey Cycle 4 (2018–2020). Using generalized linear model (GLM) analysis, we analyzed the association of 72 variables with 3 liver function indices (aspartate aminotransferase [AST], alanine aminotransferase [ALT], and gamma glutamyl transferase [GGT]). Finally, we visualized our results with Manhattan plot.
-
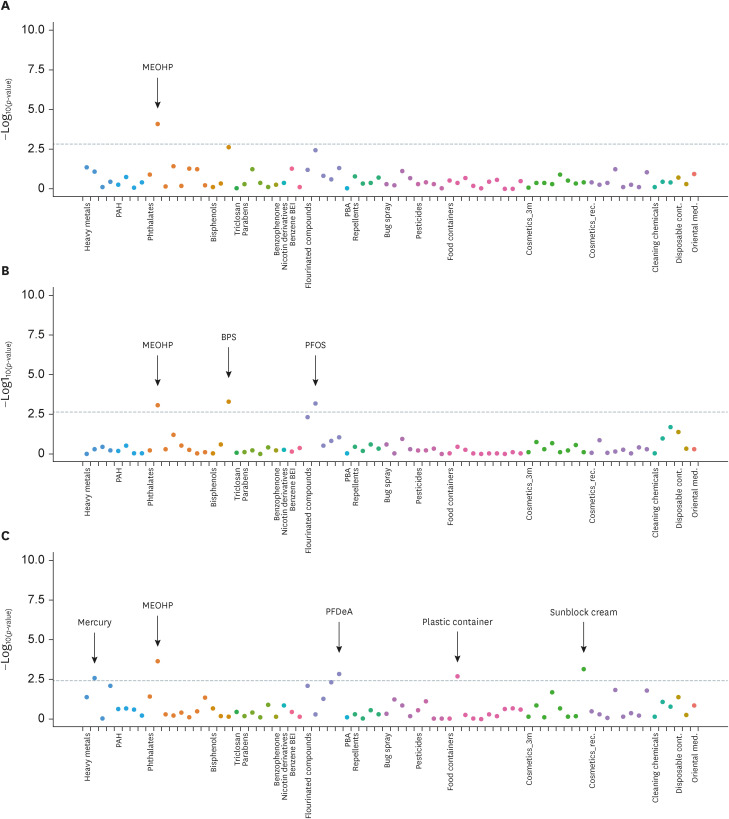
Results In GLM analysis, perfluorooctanesulfonate were positively associated with ALT (odds ratio [OR]: 2.2; 95% confidence interval [CI]: 1.39–3.46; p adjusted = 0.0147) and perfluorodecanoic acid showed positive association with GGT (OR: 2.73; 95% CI: 1.36–5.5; p adjusted = 0.0256). Plasma mercury showed positive association with GGT (OR: 1.45; 95% CI: 1.14–1.84; p adjusted = 0.0315). Using a plastic container while keeping food in the refrigerator was associated with elevated GGT compared to using a glass container (OR: 1.51; 95% CI: 1.16–1.95; p adjusted = 0.0153). 2-ethyl-5-oxohexyl phthalate, showed a negative trend with all 3 indices, with AST (OR: 0.54; 95% CI: 0.39–0.73; p adjusted = 0.00357), ALT (OR: 0.5; 95% CI: 0.34–0.75; p adjusted = 0.036), GGT (OR: 0.55; 95% CI: 0.4–0.76; p adjusted = 0.00697). Bisphenol S and frequent use of sunblock cream showed negative association with ALT (OR: 0.77; 95% CI: 0.66–0.89), and GGT (OR: 0.25; 95% CI: 0.11–0.55), respectively.
-
Conclusions We conducted an exploratory study on environmental exposure and human liver function. By using EWAS methodology, we identified 7 factors that could have potential association with liver function.
BACKGROUND
METHODS
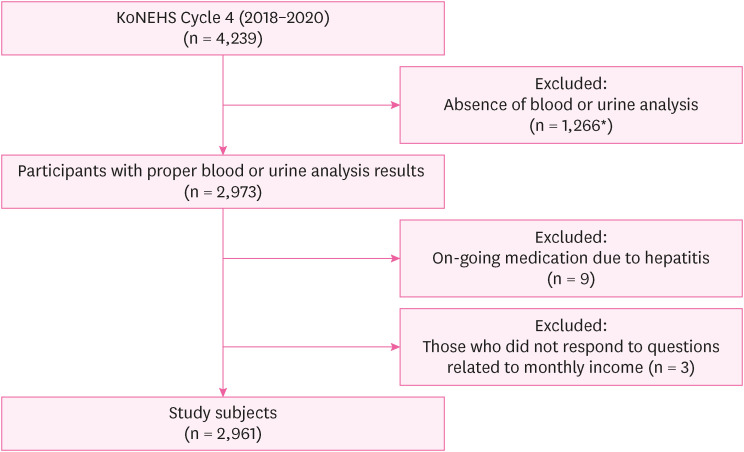
Flow of study participants selection.
RESULTS
General characteristics of subjects
Summary of statistically significant associations with abnormal liver function group
Manhattan plot of EWAS of environmentally hazardous chemicals for elevated liver enzymes. (A) Manhattan plot of EWAS for elevated aspartate aminotransferase. (B) Manhattan plot of EWAS for elevated alanine aminotransferase. (C) Manhattan plot of EWAS for elevated gamma glutamyl transferase. X-axis shows the groups of categorized chemicals. Y-axis shows −log10(p-value) of the generalized linear model analysis with each variables with elevated liver enzymes. Each categorized group was represented as same color of dots. The statistically significant cut-off values of false discovery rate < 0.05 were represented as grey dashed line.
DISCUSSION
CONCLUSIONS
ACKNOWLEDGEMENTS
Abbreviations
AST
ALT
BMI
BPA
BPS
CI
cont.
DEHP
EWAS
FDR
GGT
GLM
GWAS
IRB
KoNEHS
MEHP
MEOHP
med.
OR
PAH
PBA
PFAS
PFDeA
PFOA
PFOS
rec.
3m
-
Funding: This research was supported by the Inha University Hospital’s Environmental Health Center for Training Environmental Medicine Professionals funded by the Ministry of Environment, Republic of Korea (2022).
-
Competing interests: The authors declare that they have no competing interests.
-
Author Contributions:
NOTES
SUPPLEMENTARY MATERIALS
Supplementary Table 1
- 1. European Union. The European environment – state and outlook 2020. Updated 2020]. Accessed December 15, 2022]. http://www.eea.europa.eu/soer/publications/soer-2020 .
- 2. World Health Organization. Preventing noncommunicable diseases (NCDs) by reducing environmental risk factors. Updated 2017]. Accessed December 15, 2022]. https://apps.who.int/iris/bitstream/handle/10665/258796/WHO-FWC-EPE-17.01-eng.pdf .
- 3. Kortenkamp A, Faust M, Scholze M, Backhaus T. Low-level exposure to multiple chemicals: reason for human health concerns? Environ Health Perspect 2007;115:Suppl 1. (Suppl 1):106–114. 18174958.ArticlePubMedPMC
- 4. Sprinkle RH, Payne-Sturges DC. Mixture toxicity, cumulative risk, and environmental justice in United States federal policy, 1980-2016: why, with much known, was little done? Environ Health 2021;20(1):104. 34535123.PubMedPMC
- 5. Patel CJ. Introduction to environment and exposome-wide association studies: a data-driven method to identify multiple environmental factors associated with phenotypes in human populations. In: Rider CV, Simmons JE, editors. Chemical Mixtures and Combined Chemical and Nonchemical Stressors. Cham, Switzerland: Springer International Publishing AG; 2018, 129–149.
- 6. Rappaport SM, Barupal DK, Wishart D, Vineis P, Scalbert A. The blood exposome and its role in discovering causes of disease. Environ Health Perspect 2014;122(8):769–774. 24659601.ArticlePubMedPMC
- 7. Wahlang B, Beier JI, Clair HB, Bellis-Jones HJ, Falkner KC, McClain CJ, et al. Toxicant-associated steatohepatitis. Toxicol Pathol 2013;41(2):343–360. 23262638.ArticlePubMedPMCPDF
- 8. Wahlang B, Jin J, Beier JI, Hardesty JE, Daly EF, Schnegelberger RD, et al. Mechanisms of environmental contributions to fatty liver disease. Curr Environ Health Rep 2019;6(3):80–94. 31134516.ArticlePubMedPMCPDF
- 9. Ledda C, Loreto C, Zammit C, Marconi A, Fago L, Matera S, et al. Non-infective occupational risk factors for hepatocellular carcinoma: a review (review). Mol Med Rep 2017;15(2):511–533. 28000892.PubMed
- 10. National Center for Environmental Health. Fourth national report on human exposure to environmental chemicals. Updated tables, March 2021: volume three: analysis of pooled serum samples for select chemicals, NHANES 2005-2016. Updated 2021]. Accessed December 15, 2022]. https://stacks.cdc.gov/view/cdc/105344 .
- 11. Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, et al. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med 2018;75(1):46–51. 29133598.ArticlePubMedPMC
- 12. Zhao M, Ge X, Xu J, Li A, Mei Y, Yin G, et al. Association between urine metals and liver function biomarkers in Northeast China: a cross-sectional study. Ecotoxicol Environ Saf 2022;231:113163. 35030523.ArticlePubMed
- 13. Chang WJ, Joe KT, Park HY, Jeong JD, Lee DH. The relationship of liver function tests to mixed exposure to lead and organic solvents. Ann Occup Environ Med 2013;25(1):5. 24472152.ArticlePubMedPMC
- 14. Cave M, Appana S, Patel M, Falkner KC, McClain CJ, Brock G. Polychlorinated biphenyls, lead, and mercury are associated with liver disease in American adults: NHANES 2003-2004. Environ Health Perspect 2010;118(12):1735–1742. 21126940.ArticlePubMedPMC
- 15. Feng C, Wang H, Lu N, Chen T, He H, Lu Y, et al. Log-transformation and its implications for data analysis. Shanghai Arch Psychiatry 2014;26(2):105–109. 25092958.PubMedPMC
- 16. Patel CJ, Bhattacharya J, Butte AJ. An environment-wide association study (EWAS) on type 2 diabetes mellitus. PLoS One 2010;5(5):e10746. 20505766.ArticlePubMedPMC
- 17. Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A 2003;100(16):9440–9445. 12883005.ArticlePubMedPMC
- 18. Benjamini Y, Cohen R. Weighted false discovery rate controlling procedures for clinical trials. Biostatistics 2017;18(1):91–104. 27445132.ArticlePubMedPMC
- 19. Hall MA, Dudek SM, Goodloe R, Crawford DC, Pendergrass SA, Peissig P, et al. Environment-wide association study (EWAS) for type 2 diabetes in the Marshfield Personalized Medicine Research Project Biobank. Pac Symp Biocomput 2014;200–211.Article
- 20. Seo MS, Lee HR, Shim JY, Kang HT, Lee YJ. Relationship between blood mercury concentrations and serum γ-glutamyltranspeptidase level in Korean adults using data from the 2010 Korean National Health and Nutrition Examination Survey. Clin Chim Acta 2014;430:160–163. 24508988.ArticlePubMed
- 21. Choi J, Bae S, Lim H, Lim JA, Lee YH, Ha M, et al. Mercury exposure in association with decrease of liver function in adults: a longitudinal study. J Prev Med Public Health 2017;50(6):377–385. 29207447.ArticlePubMedPMCPDF
- 22. Lim JS, Yang JH, Chun BY, Kam S, Jacobs DR Jr, Lee DH. Is serum gamma-glutamyltransferase inversely associated with serum antioxidants as a marker of oxidative stress? Free Radic Biol Med 2004;37(7):1018–1023. 15336318.PubMed
- 23. Farina M, Avila DS, da Rocha JB, Aschner M. Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochem Int 2013;62(5):575–594. 23266600.ArticlePubMedPMC
- 24. Hu XF, Singh K, Chan HM. Mercury exposure, blood pressure, and hypertension: a systematic review and dose-response meta-analysis. Environ Health Perspect 2018;126(7):076002. 30073953.ArticlePubMedPMC
- 25. He K, Xun P, Liu K, Morris S, Reis J, Guallar E. Mercury exposure in young adulthood and incidence of diabetes later in life: the CARDIA Trace Element Study. Diabetes Care 2013;36(6):1584–1589. 23423697.PubMedPMC
- 26. Mahaffey KR, Mergler D. Blood levels of total and organic mercury in residents of the upper St. Lawrence River basin, Québec: association with age, gender, and fish consumption. Environ Res 1998;77(2):104–114. 9600803.ArticlePubMed
- 27. Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 2006;296(15):1885–1899. 17047219.ArticlePubMed
- 28. Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 2011;7(4):513–541. 21793199.ArticlePubMedPMC
- 29. Costello E, Rock S, Stratakis N, Eckel SP, Walker DI, Valvi D, et al. Exposure to per- and polyfluoroalkyl substances and markers of liver injury: a systematic review and meta-analysis. Environ Health Perspect 2022;130(4):46001. 35475652.ArticlePubMedPMC
- 30. Das KP, Wood CR, Lin MT, Starkov AA, Lau C, Wallace KB, et al. Perfluoroalkyl acids-induced liver steatosis: effects on genes controlling lipid homeostasis. Toxicology 2017;378:37–52. 28049043.ArticlePubMedPMC
- 31. Bagley BD, Chang SC, Ehresman DJ, Eveland A, Zitzow JD, Parker GA, et al. Perfluorooctane sulfonate-induced hepatic steatosis in male Sprague Dawley rats is not attenuated by dietary choline supplementation. Toxicol Sci 2017;160(2):284–298. 28973659.ArticlePubMed
- 32. Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 2007;99(2):366–394. 17519394.ArticlePubMed
- 33. Abdellatif A, Al-Tonsy AH, Awad ME, Roberfroid M, Khan MN. Peroxisomal enzymes and 8-hydroxydeoxyguanosine in rat liver treated with perfluorooctanoic acid. Dis Markers 2003-2004;19(1):19–25.ArticlePubMedPMCPDF
- 34. Bjork JA, Butenhoff JL, Wallace KB. Multiplicity of nuclear receptor activation by PFOA and PFOS in primary human and rodent hepatocytes. Toxicology 2011;288(1-3):8–17. 21723365.ArticlePubMed
- 35. Elcombe CR, Elcombe BM, Foster JR, Farrar DG, Jung R, Chang SC, et al. Hepatocellular hypertrophy and cell proliferation in Sprague-Dawley rats following dietary exposure to ammonium perfluorooctanoate occurs through increased activation of the xenosensor nuclear receptors PPARα and CAR/PXR. Arch Toxicol 2010;84(10):787–798. 20614104.ArticlePubMedPDF
- 36. Vered G, Shenkar N. Monitoring plastic pollution in the oceans. Curr Opin Toxicol 2021;27:60–68.Article
- 37. Proshad R, Kormoker T, Islam S, Haque MA, Rhaman M, Mithu MR. Toxic effects of plastic on human health and environment: aconsequences of health risk assessment in Bangladesh. Int J Heal 2018;6(1):1–5.ArticlePDF
- 38. Katsikantami I, Sifakis S, Tzatzarakis MN, Vakonaki E, Kalantzi OI, Tsatsakis AM, et al. A global assessment of phthalates burden and related links to health effects. Environ Int 2016;97(97):212–236. 27669632.ArticlePubMed
- 39. Park O, Park JT, Chi Y, Kwak K. Association of phthalates and early menarche in Korean adolescent girls from Korean National Environmental Health Survey (KoNEHS) 2015-2017. Ann Occup Environ Med 2021;33(1):e4. 34754465.ArticlePubMedPMCPDF
- 40. Rowdhwal SS, Chen J. Toxic effects of di-2-ethylhexyl phthalate: an overview. BioMed Res Int 2018;2018:1750368. 29682520.ArticlePubMedPMCPDF
- 41. Ferguson KK, Loch-Caruso R, Meeker JD. Urinary phthalate metabolites in relation to biomarkers of inflammation and oxidative stress: NHANES 1999-2006. Environ Res 2011;111(5):718–726. 21349512.ArticlePubMedPMC
- 42. Erkekoglu P, Rachidi W, Yuzugullu OG, Giray B, Favier A, Ozturk M, et al. Evaluation of cytotoxicity and oxidative DNA damaging effects of di(2-ethylhexyl)-phthalate (DEHP) and mono(2-ethylhexyl)-phthalate (MEHP) on MA-10 Leydig cells and protection by selenium. Toxicol Appl Pharmacol 2010;248(1):52–62. 20659492.ArticlePubMed
- 43. Hauser R. Urinary phthalate metabolites and semen quality: a review of a potential biomarker of susceptibility. Int J Androl 2008;31(2):112–117. 18067563.ArticlePubMed
- 44. Meeker JD, Calafat AM, Hauser R. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect 2007;115(7):1029–1034. 17637918.ArticlePubMedPMC
- 45. Qin J, Ru S, Wang W, Hao L, Ru Y, Wang J, et al. Long-term bisphenol S exposure aggravates non-alcoholic fatty liver by regulating lipid metabolism and inducing endoplasmic reticulum stress response with activation of unfolded protein response in male zebrafish. Environ Pollut 2020;263(Pt B):114535. 32283406.ArticlePubMed
- 46. Al-Eitan LN, Aljamal HA, Alkhatib RQ. Gas chromatographic-mass spectrometric analysis of sunscreens and their effects on mice liver and kidney enzyme function. Clin Cosmet Investig Dermatol 2018;12:11–21.PubMedPMC
- 47. Gorman S, Black LJ, Feelisch M, Hart PH, Weller R. Can skin exposure to sunlight prevent liver inflammation? Nutrients 2015;7(5):3219–3239. 25951129.ArticlePubMedPMC
REFERENCES
REFERENCES
Figure & Data
REFERENCES
Citations

- Exposome-wide association study of cognitive function in US older adults using the NHANES data
Hyuna Jang, Jiyun Lee, Vy Kim Nguyen, Hyeong-Moo Shin
Exposome.2025;[Epub] CrossRef - Liver Toxicity Induced by Exposure to Bisphenol Analogs at Environmentally Relevant Levels: Insights from a Literature Review on Multiple Species
Tai L. Guo, Fatma Eldefrawy, Kevin M. Guo
Livers.2025; 5(2): 24. CrossRef - Sex Differences in the Association Between the Korean Healthy Eating Index and Liver Enzymes Among Korean Adults
Seong-Uk Baek, Jin-Ha Yoon
Nutrients.2025; 17(14): 2372. CrossRef - Association Between Heavy Metals Exposure and Elevated High-Sensitivity C-Reactive Protein: Mediating Role of Body Mass Index
Seong-Uk Baek, Jin-Ha Yoon
Biomolecules.2025; 15(11): 1491. CrossRef - Metals exposure and biomarkers of liver damage: a systematic review and meta-analysis of observational studies
Ibrahim Issah, Serwaa A. Bawua, John Arko-Mensah, Mabel S. Duah, Shirley V. Simpson, Thomas P. Agyekum, Olalekan A. Uthman, Julius N. Fobil
Reviews on Environmental Health.2025;[Epub] CrossRef - Association between gut microbiota composition and liver dysfunction in school-aged children exposed to triclosan
Aijing Li, Menglong Li, Wang Xu, Yanhui Zhang, Shulan Yin, Yating Yu, Shufa Zheng, Yifei Hu, Maoyong Song
Journal of Environmental Sciences.2025;[Epub] CrossRef
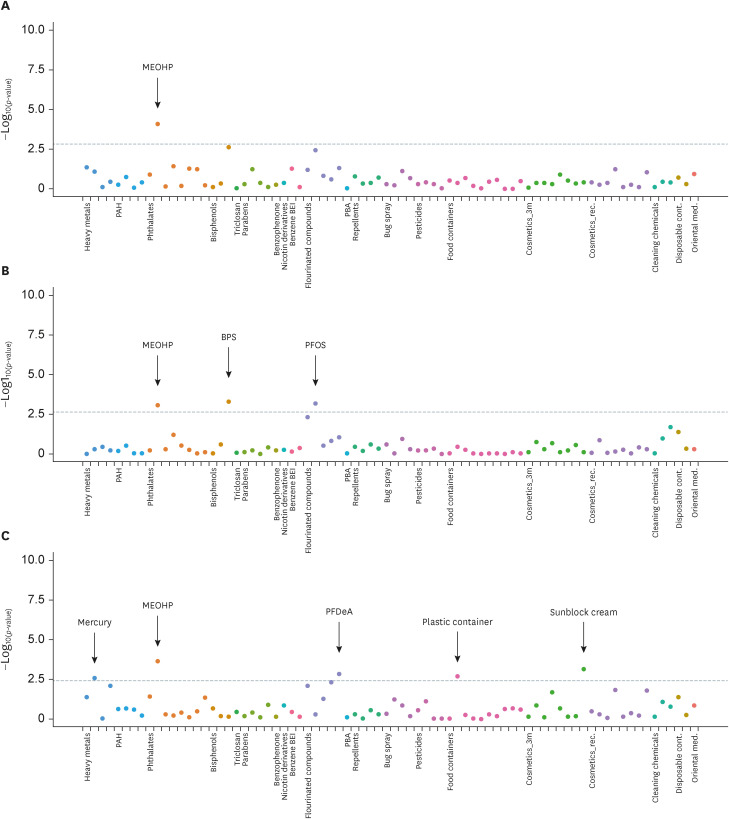
Fig. 1
Fig. 2
| Characteristics | All (n = 2,961) | Dysfunction group | ||||||
|---|---|---|---|---|---|---|---|---|
| AST (n = 370) | ALT (n = 195) | GGT (n = 342) | ||||||
| Sex | < 0.001*** | < 0.001*** | < 0.01** | |||||
| Male | 1,289 (43.5) | 215 (58.1) | 134 (68.7) | 175 (51.2) | ||||
| Female | 1,672 (56.5) | 155 (41.9) | 61 (31.3) | 167 (48.8) | ||||
| Age | 52.01 ± 14.0 | 56.1 ± 13.6 | < 0.001*** | 47.8 ± 14.9 | < 0.001*** | 53.8 ± 12.8 | 0.092 | |
| BMI | < 0.001*** | < 0.001*** | < 0.001*** | |||||
| < 23 | 872 (29.5) | 67 (18.1) | 10 (5.1) | 60 (17.5) | ||||
| 23–25 | 679 (22.9) | 67 (18.1) | 32 (16.4) | 68 (19.9) | ||||
| ≥ 25 | 1,410 (47.6) | 236 (63.8) | 153 (78.5) | 214 (62.6) | ||||
| Drinking | < 0.001*** | < 0.05* | < 0.001*** | |||||
| None | 912 (30.8) | 109 (29.5) | 46 (23.6) | 72 (21.1) | ||||
| Group 1 | 473 (16.0) | 46 (12.4) | 23 (11.8) | 38 (11.1) | ||||
| Group 2 | 524 (17.7) | 52 (14.1) | 38 (19.5) | 35 (10.2) | ||||
| Group 3 | 588 (19.9) | 76 (20.5) | 48 (24.6) | 70 (20.5) | ||||
| Group 4 | 301 (10,2) | 53 (14.3) | 26 (13.3) | 72 (21.1) | ||||
| Group 5 | 163 (5.5) | 34 (9.2) | 14 (7.2) | 55 (16.1) | ||||
| Smoking | < 0.001*** | < 0.001*** | < 0.001*** | |||||
| Never | 1,905 (64.3) | 193 (52.2) | 94 (48.2) | 189 (55.3) | ||||
| Quit | 593 (20.0) | 101 (27.3) | 44 (22.6) | 66 (19.3) | ||||
| Current | 463 (15.7) | 76 (20.5) | 57 (29.2) | 87 (25.4) | ||||
| Monthly income | < 0.001*** | 0.301 | 0.097 | |||||
| Group 1 | 1,343 (45.4) | 208 (56.2) | 81 (41.5) | 170 (49.7) | ||||
| Group 2 | 1,618 (54.6) | 162 (43.8) | 114 (58.5) | 172 (50.3) | ||||
| Hazardous substances | OR (95% CI) | FDR | |||
|---|---|---|---|---|---|
| Abnormal AST | |||||
| Phthalates | |||||
| MEOHP (µg/L) | 0.54 (0.39–0.73) | 8.40 × 10−5 | 3.57 × 10−3** | ||
| Abnormal ALT | |||||
| Phthalates | |||||
| MEOHP (µg/L) | 0.5 (0.34–0.75) | 8.46 × 10−4 | 3.60 × 10−2* | ||
| Bisphenols | |||||
| BPS (µg/L) | 0.77 (0.66–0.89) | 5.32 × 10−4 | 1.51 × 10−2* | ||
| Perfluorinated compounds | |||||
| PFOS (µg/L) | 2.2 (1.39–3.46) | 6.93 × 10−4 | 1.47 × 10−2* | ||
| Abnormal GGT | |||||
| Heavy metals | |||||
| Mercury (plasma) (µg/L) | 1.45 (1.14–1.84) | 2.59 × 10−3 | 3.15 × 10−2* | ||
| Phthalates | |||||
| MEOHP (µg/L) | 0.55 (0.4–0.76) | 2.46 × 10−4 | 6.97 × 10−3** | ||
| Perfluorinated compounds | |||||
| PFDeA (µg/L) | 2.73 (1.36–5.5) | 1.50 × 10−3 | 2.56 × 10−2* | ||
| Food container | |||||
| Plastic (compared to glass) | 1.51 (1.16–1.95) | 2.13 × 10−3 | 1.53 × 10−2* | ||
| Cosmetics | |||||
| Wearing sun block cream (≥ 1–2 times per week) | 0.25 (0.11–0.55) | 7.18 × 10−4 | 3.02 × 10−2* | ||
Values are presented as number (%) or mean ± standard deviation.
Monthly income (average monthly household income over the past year): Group 1, less than 3 million won; Group 2, equal or more than 3 million won.
Drinking: None, people who answered they do not drink; Group 1, people who drinks less than once in a month; Group 2, people who drinks 1–2 times in a month; Group 3, people who drinks 1–2 times in a week; Group 4, people who drinks more than 3 times in a week; Group 5, people who drinks almost everyday.
BMI: body mass index; AST: aspartate aminotransferase; ALT: alanine aminotransferase; GGT: gamma glutamyl transferase.
*
Among 72 variables, one variable for AST, 3 variables for ALT, 5 variables for GGT were identified as statistically significant association. To correct possible type I error,
OR: odds ratio; CI: confidence interval; FDR: false discovery rate; AST: aspartate aminotransferase; ALT: alanine aminotransferase; GGT: gamma glutamyl transferase; MEOHP: 2-ethyl-5-oxohexyl phthalate; BPS: bisphenol S; PFOS: perfluorooctanesulfonate; PFDeA: perfluorodecanoic acid.
*FDR < 0.05; **FDR < 0.01.
 KSOEM
KSOEM

 Cite
Cite