Articles
- Page Path
- HOME > Ann Occup Environ Med > Volume 29; 2017 > Article
- Research Article Association of arsenobetaine with beta-cell function assessed by homeostasis model assessment (HOMA) in nondiabetic Koreans: data from the fourth Korea National Health and Nutrition Examination Survey (KNHANES) 2008-2009
- Kiook Baek1, Namhoon Lee1, Insung Chung1,2
-
Annals of Occupational and Environmental Medicine 2017;29:31.
DOI: https://doi.org/10.1186/s40557-017-0181-0
Published online: July 10, 2017
1Division of Occupational and Environmental Medicine, Keimyung University Dongsan Medical Center, Daegu, South Korea
2Department of Preventive Medicine, Keimyung University School of Medicine, Daegu, South Korea
© The Author(s). 2017
Open AccessThis article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Abstract
-
Background Arsenic is known as an endocrine disruptor that people are exposed to through various sources such as drinking water and indigestion of marine products. Although some epidemiological and animal studies have reported a correlation between arsenic exposure and diabetes development, there are limited studies regarding the toxic effects of organic arsenic including arsenobetaine on the human body. Here, we analyzed the association between urine arsenobetaine and the homeostasis model assessment of β-cell function (HOMA-β), which is an index for predicting diabetes development and reflecting the function of pancreatic β-cells.
-
Methods In the fourth Korea National Health and Nutrition Examination Survey (KNHANES), health and nutrition surveys and screening tests were performed. Of the total survey population, people with confirmed values for urine total arsenic and arsenobetaine were included, and known diabetic patients were excluded. A total 369 participants were finally included in the study. We collected surveys on health, height, body weight, body mass index, blood mercury level, fasting glucose level, and serum insulin level and calculated HOMA index. Owing to sexual discrepancy, we performed sexually stratified analysis.
-
Results Urine total arsenic and total arsenic minus arsenobetaine was not associated with HOMA-IR and HOMA-β in univariate analysis or in sexually stratified analysis. However, urine arsenobetaine showed a statistically significant relationship with HOMA-β in univariate analysis, and only male participants showed a significant correlation in sexually stratified analysis. In the analysis adjusted for age, BMI, smoking, alcohol drinking, physical activity and blood mercury, the HOMA-β value in the group below the 25th percentile of arsenobetaine was significantly higher than the group between 50 and 75th percentile, while no difference was shown for HOMA-IR. In sexually stratified analysis, The value of HOMA-β was significantly higher in male participants with below the 25th percentile urine arsenobetaine than the group between 25 and 50th and between 50 and 75th, while no difference was shown for HOMA-IR. However, female participants did not demonstrate a relationship between HOMA–IR, HOMA-β and urine arsenobetaine.
-
Conclusion This study revealed the association between urine arsenobetaine and pancreatic β-cell function assessed by HOMA-β in the normal population (without diabetes), especially in males, despite adjusting for factors affecting pancreatic β-cell function and diabetes.
-
Electronic supplementary material The online version of this article (doi:10.1186/s40557-017-0181-0) contains supplementary material, which is available to authorized users.
Background
Methods
Results
Discussion
Conclusion
Additional files
Additional file 1: Table S1.
Acknowledgements
Abbreviations
ANOVA
C.I
DMA
DMAA
HOMA
ICP-MS
KNHANES
MA
MMA
ROS
TMAO
-
Electronic supplementary material
The online version of this article (doi:10.1186/s40557-017-0181-0) contains supplementary material, which is available to authorized users.
NOTES
- 1. Gebel TW. Arsenic and drinking water contamination. Science 1999;283(5407):1458–1459. 10.1126/science.283.5407.1455e. 10206874.ArticlePubMed
- 2. Edmonds J, et al. Arsenic transformations in short marine food chains studied by HPLC-ICP MS. Appl Organomet Chem 1997;11(4):281–287. 10.1002/(SICI)1099-0739(199704)11:4<281::AID-AOC581>3.0.CO;2-S.Article
- 3. De Coster S, van Larebeke N. Endocrine-disrupting chemicals: associated disorders and mechanisms of action. J Environ Public Health 2012;2012:713696. 10.1155/2012/713696. 22991565.ArticlePubMedPMC
- 4. Matthews D, et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28(7):412–419. 10.1007/BF00280883. 3899825.ArticlePubMedPDF
- 5. Navas-Acien A, et al. Rejoinder: arsenic exposure and prevalence of type 2 diabetes: updated findings from the National Health Nutrition and Examination survey, 2003-2006. Epidemiology 2009;20(6):816–820. 10.1097/EDE.0b013e3181afef88. 19713856.PubMedPMC
- 6. Navas-Acien A, et al. Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiologic evidence. Environ Health Perspect 2006;114(5):641–648. 10.1289/ehp.8551. 16675414.ArticlePubMed
- 7. Tseng CH. The potential biological mechanisms of arsenic-induced diabetes mellitus. Toxicol Appl Pharmacol 2004;197(2):67–83. 10.1016/j.taap.2004.02.009. 15163543.ArticlePubMed
- 8. Becker A, Axelrad D. Arsenic and type 2 diabetes: commentary on association of inorganic arsenic exposure with type 2 diabetes mellitus: a meta-analysis by Wang et al. J Epidemiol Community Health 2014;68(5):393–5. 10.1136/jech-2013-203463. 24558058.ArticlePubMed
- 9. Steinmaus C, et al. Low-level population exposure to inorganic arsenic in the United States and diabetes mellitus: a reanalysis. Epidemiology 2009;20(6):807–815. 10.1097/EDE.0b013e3181b0fd29. 19652600.PubMed
- 10. Buchet J-P, Pauwels J, Lauwerys R. Assessment of exposure to inorganic arsenic following ingestion of marine organisms by volunteers. Environ Res 1994;66(1):44–51. 10.1006/enrs.1994.1043. 8013437.ArticlePubMed
- 11. Newcombe C, et al. Accumulation or production of arsenobetaine in humans? J Environ Monit 2010;12(4):832–837. 10.1039/b921588c. 20383363.ArticlePubMed
- 12. Harrington CF, Brima EI, Jenkins RO. Biotransformation of arsenobetaine by microorganisms from the human gastrointestinal tract. Chem Speciation Bioavailability 2008;20(3):173–180. 10.3184/095422908X347278.Article
- 13. Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 1990;5(1):46–51. 10.1080/1047322X.1990.10389587.Article
- 14. Nehls GJ, Akland GG. Procedures for handling aerometric data. J Air Pollut Control Assoc 1973;23(3):180–184. 10.1080/00022470.1973.10469762.Article
- 15. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27(6):1487–1495. 10.2337/diacare.27.6.1487. 15161807.ArticlePubMedPDF
- 16. Chiu KC, et al. Beta cell function declines with age in glucose tolerant Caucasians. Clin Endocrinol 2000;53(5):569–575. 10.1046/j.1365-2265.2000.01132.x.ArticlePubMedPDF
- 17. Ostgren C, et al. Associations between smoking and beta-cell function in a non-hypertensive and non-diabetic population. Skaraborg hypertension and diabetes project. Diabetic Med 2000;17(6):445–450. 10.1046/j.1464-5491.2000.00294.x. 10975213.PubMed
- 18. Howard AA, Arnsten JH, Gourevitch MN. Effect of alcohol consumption on diabetes mellitus: a systematic review. Ann Intern Med 2004;140(3):211–219. 10.7326/0003-4819-140-6-200403160-00011. 14757619.ArticlePubMed
- 19. Chen G, et al. Overweight, obesity, and their associations with insulin resistance and β-cell function among Chinese: a cross-sectional study in China. Metabolism 2010;59(12):1823–1832. 10.1016/j.metabol.2010.06.009. 20655552.Article
- 20. Riddell MC, Sigal RJ. Physical activity, exercise and diabetes. Can J Diabetes 2013;37(6):359–360. 10.1016/j.jcjd.2013.10.001. 24321713.ArticlePubMed
- 21. Rhee SY, et al. Arsenic exposure and prevalence of diabetes mellitus in Korean adults. J Korean Med Sci 2013;28(6):861–868. 10.3346/jkms.2013.28.6.861. 23772150.ArticlePubMedPMCPDF
- 22. Navas-Acien A, et al. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA 2008;300(7):814–822. 10.1001/jama.300.7.814. 18714061.ArticlePubMed
- 23. Maull EA, et al. Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ Health Perspect 2012;120(12):1658. 10.1289/ehp.1104579. 22889723.ArticlePubMedPMC
- 24. Park SK, et al. Arsenic exposure is associated with diminished insulin sensitivity in non-diabetic Amish adults. Diabetes Metab Res Rev 2016;32(6):565–571. 10.1002/dmrr.2769. 26663816.ArticlePubMedPMC
- 25. Islam R, et al. Association between type 2 diabetes and chronic arsenic exposure in drinking water: a cross sectional study in Bangladesh. Environ Health 2012;11:38. 10.1186/1476-069X-11-38. 22676249.ArticlePubMedPMCPDF
- 26. Kim Y, Lee B-K. Association between urinary arsenic and diabetes mellitus in the Korean general population according to KNHANES 2008. Sci Total Environ 2011;409(19):4054–4062. 10.1016/j.scitotenv.2011.06.003. 21723589.ArticlePubMed
- 27. Lai MS, et al. Ingested inorganic arsenic and prevalence of diabetes mellitus. Am J Epidemiol 1994;139(5):484–492. 10.1093/oxfordjournals.aje.a117031. 8154472.ArticlePubMed
- 28. Chen Y, et al. No association between arsenic exposure from drinking water and diabetes mellitus: a cross-sectional study in Bangladesh. Environ Health Perspect 2010;118(9):1299–1305. 10.1289/ehp.0901559. 20813654.Article
- 29. Zierold KM, Knobeloch L, Anderson H. Prevalence of chronic diseases in adults exposed to arsenic-contaminated drinking water. Am J Public Health 2004;94(11):1936–1937. 10.2105/AJPH.94.11.1936. 15514231.ArticlePubMedPMC
- 30. Peng Q, Harlow SD, Park SK. Urinary arsenic and insulin resistance in US adolescents. Int J Hyg Environ Health 2015;218(4):407–413. 10.1016/j.ijheh.2015.03.006. 25845984.ArticlePubMedPMC
- 31. Ruiz-Navarro ML, et al. Urine arsenic concentrations in healthy adults as indicators of environmental contamination: relation with some pathologies. Sci Total Environ 1998;216(1-2):55–61. 10.1016/S0048-9697(98)00136-3. 9618928.ArticlePubMed
- 32. Hectors T, et al. Environmental pollutants and type 2 diabetes: a review of mechanisms that can disrupt beta cell function. Diabetologia 2011;54(6):1273–1290. 10.1007/s00125-011-2109-5. 21442161.ArticlePubMedPDF
- 33. Izquierdo-Vega JA, et al. Diabetogenic effects and pancreatic oxidative damage in rats subchronically exposed to arsenite. Toxicol Lett 2006;160(2):135–142. 10.1016/j.toxlet.2005.06.018. 16111841.ArticlePubMed
- 34. Paul DS, et al. Examination of the effects of arsenic on glucose homeostasis in cell culture and animal studies: development of a mouse model for arsenic-induced diabetes. Toxicol Appl Pharmacol 2007;222(3):305–314. 10.1016/j.taap.2007.01.010. 17336358.ArticlePubMedPMC
- 35. Díaz-Villaseñor A, et al. Arsenic-induced alteration in the expression of genes related to type 2 diabetes mellitus. Toxicol Appl Pharmacol 2007;225(2):123–133. 10.1016/j.taap.2007.08.019. 17936320.ArticlePubMed
- 36. Díaz-Villaseñor A, et al. Sodium arsenite impairs insulin secretion and transcription in pancreatic β-cells. Toxicol Appl Pharmacol 2006;214(1):30–34. 10.1016/j.taap.2005.11.015. 16413591.ArticlePubMed
- 37. Fu J, et al. Low-level arsenic impairs glucose-stimulated insulin secretion in pancreatic beta cells: involvement of cellular adaptive response to oxidative stress. Environ Health Perspect 2010;118(6):864. 10.1289/ehp.0901608. 20100676.ArticlePubMedPMC
- 38. Gresser MJ. ADP-arsenate. Formation by submitochondrial particles under phosphorylating conditions. J Biol Chem 1981;256(12):5981–5983. 7240187.ArticlePubMed
- 39. Song Y, et al. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and risk of diabetes in a multiethnic cohort of women: the Women’s Health Initiative observational study. Diabetes Care 2007;30(7):1747–1752. 10.2337/dc07-0358. 17468352.ArticlePubMedPDF
- 40. Choi E-S, et al. Insulin sensitivity and insulin secretion determined by homeostasis model assessment and future risk of diabetes mellitus in Korean men. Korean Diabetes J 2008;32(6):498–505. 10.4093/kdj.2008.32.6.498.
- 41. Bonora E, et al. Population-based incidence rates and risk factors for type 2 diabetes in white individuals: the Bruneck study. Diabetes 2004;53(7):1782–1789. 10.2337/diabetes.53.7.1782. 15220202.Article
- 42.
- 43. Kaise T, Fukui S. The chemical form and acute toxicity of arsenic compounds in marine organisms. Appl Organomet Chem 1992;6(2):155–160. 10.1002/aoc.590060208.Article
- 44. Borak J, Hosgood HD. Seafood arsenic: implications for human risk assessment. Regul Toxicol Pharmacol 2007;47(2):204–212. 10.1016/j.yrtph.2006.09.005. 17092619.ArticlePubMed
- 45. Hygienists(ACGIH), A.C.o.G.I. Documentation of the threshold limit values and biological exposure indices (7th Ed.). 2007, Cincinnati: ACGIH.
- 46. Goessler W, et al. Can humans metabolize arsenic compounds to arsenobetaine? Appl Organomet Chem 1997;11(4):327–335. 10.1002/(SICI)1099-0739(199704)11:4<327::AID-AOC589>3.0.CO;2-Q.Article
- 47. Brown RM, et al. Human metabolism of arsenobetaine ingested with fish. Hum Exp Toxicol 1990;9(1):41–46. 10.1177/096032719000900109. 2328148.ArticlePubMedPDF
- 48. Yamauchi H, Kaise T, Yamamura Y. Metabolism and excretion of orally administered arsenobetaine in the hamster. Bull Environ Contam Toxicol 1986;36(1):350–355. 10.1007/BF01623519. 3955245.PubMed
- 49. Yamauchi H, Yamamura Y. Metabolism and excretion of orally ingested trimethylarsenic in man. Bull Environ Contam Toxicol 1984;32(6):682–687. 10.1007/BF01607556. 6743858.ArticlePubMedPDF
- 50. Chavez-Capilla T, et al. Bioaccessibility and degradation of naturally occurring arsenic species from food in the human gastrointestinal tract. Food Chem 2016;212:189–197. 10.1016/j.foodchem.2016.05.163. 27374523.ArticlePubMed
- 51. Wild S, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27(5):1047–1053. 10.2337/diacare.27.5.1047. 15111519.ArticlePDF
- 52. Louet JF, LeMay C, Mauvais-Jarvis F. Antidiabetic actions of estrogen: insight from human and genetic mouse models. Curr Atheroscler Rep 2004;6(3):180–185. 10.1007/s11883-004-0030-9. 15068742.ArticlePubMedPDF
- 53. Nadal A, et al. The pancreatic beta-cell as a target of estrogens and xenoestrogens: implications for blood glucose homeostasis and diabetes. Mol Cell Endocrinol 2009;304(1-2):63–68. 10.1016/j.mce.2009.02.016. 19433249.PubMed
- 54. Alonso-Magdalena P, et al. Pancreatic insulin content regulation by the estrogen receptor ERα. PLoS One 2008;3(4):e2069. 10.1371/journal.pone.0002069. 18446233.ArticlePubMedPMC
- 55. Le May C, et al. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci U S A 2006;103(24):9232–9237. 10.1073/pnas.0602956103. 16754860.PubMedPMC
- 56. Vahter M, et al. Gender differences in the disposition and toxicity of metals. Environ Res 2007;104(1):85–95. 10.1016/j.envres.2006.08.003. 16996054.ArticlePubMed
- 57.
- 58. Loffredo CA, et al. Variability in human metabolism of arsenic. Environ Res 2003;92(2):85–91. 10.1016/S0013-9351(02)00081-6. 12854687.ArticlePubMed
- 59. Tsai SM, Wang TN, Ko YC. Mortality for certain diseases in areas with high levels of arsenic in drinking water. Arch Environ Health 1999;54(3):186–193. 10.1080/00039899909602258. 10444040.PubMed
- 60. Huang CF, et al. Arsenic exposure and glucose intolerance/insulin resistance in estrogen-deficient female mice. Environ Health Perspect 2015;123(11):1138–1144. 10.1289/ehp.1408663. 25859628.ArticlePubMedPMC
- 61. Watson WH, Yager JD. Arsenic: extension of its endocrine disruption potential to interference with estrogen receptor-mediated signaling. Toxicol Sci 2007;98(1):1–4. 10.1093/toxsci/kfm111. 17650541.PubMed
- 62. Lenky CC, McEntyre CJ, Lever M. Measurement of marine osmolytes in mammalian serum by liquid chromatography-tandem mass spectrometry. Anal Biochem 2012;420(1):7–12. 10.1016/j.ab.2011.09.013. 21982861.ArticlePubMed
- 63.
- 64. Craig SA. Betaine in human nutrition. Am J Clin Nutr 2004;80(3):539–549. 15321791.PubMed
- 65. Best L, Miley HE, Yates AP. Activation of an anion conductance and beta-cell depolarization during hypotonically induced insulin release. Exp Physiol 1996;81(6):927–933. 10.1113/expphysiol.1996.sp003993. 8960699.PubMed
- 66. Best L, Speake T, Brown P. Functional characterisation of the volume-sensitive anion channel in rat pancreatic beta-cells. Exp Physiol 2001;86(2):145–150. 10.1113/eph8602118. 11429628.PubMed
- 67. Nkondjock A, Receveur O. Fish-seafood consumption, obesity, and risk of type 2 diabetes: an ecological study. Diabetes Metab 2003;29(6):635–642. 10.1016/S1262-3636(07)70080-0. 14707894.ArticlePubMed
- 68. Patel PS, et al. Association between type of dietary fish and seafood intake and the risk of incident type 2 diabetes. Diabetes Care 2009;32(10):1857–1863. 10.2337/dc09-0116. 19592633.PubMedPMC
- 69. Navas-Acien A, et al. Seafood intake and urine concentrations of total arsenic, dimethylarsinate and arsenobetaine in the US population. Environ Res 2011;111(1):110–118. 10.1016/j.envres.2010.10.009. 21093857.ArticlePubMed
- 70. Gamble MV, et al. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect 2005;113(12):1683–1688. 10.1289/ehp.8084. 16330347.PubMedPMC
- 71. Ahsan H, et al. Arsenic metabolism, genetic susceptibility, and risk of premalignant skin lesions in Bangladesh. Cancer Epidemiol Prevent Biomarkers 2007;16(6):1270–1278. 10.1158/1055-9965.EPI-06-0676.ArticlePDF
REFERENCES
Figure & Data
REFERENCES
Citations

- Risk assessment of complex organoarsenic species in food
Helle Katrine Knutsen, Agneta Åkesson, Vasileios Bampidis, Margherita Bignami, Laurent Bodin, James Kevin Chipman, Gisela Degen, Antonio Hernández‐Jerez, Tim Hofer, Christer Hogstrand, Stefano Landi, Jean‐Charles Leblanc, Kyriaki Machera, Evangelia Ntzani
EFSA Journal.2024;[Epub] CrossRef - Research for type 2 diabetes mellitus in endemic arsenism areas in central China: role of low level of arsenic exposure and KEAP1 rs11545829 polymorphism
Chenlu Fan, Zaihong Zhan, Xin Zhang, Qun Lou, Ning Guo, Mengyao Su, Yue Gao, Ming Qin, Liaowei Wu, Wei Huang, Meichen Zhang, Fanshuo Yin, Yanhui Wu, Jingbo Pi, Yuanyuan Xu, Yanmei Yang, Yanhui Gao
Archives of Toxicology.2022; 96(6): 1673. CrossRef - Arsenic exposure during pregnancy and postpartum maternal glucose tolerance: evidence from Bangladesh
Abby F. Fleisch, Sudipta Kumer Mukherjee, Subrata K. Biswas, John F. Obrycki, Sheikh Muhammad Ekramullah, D. M. Arman, Joynul Islam, David C. Christiani, Maitreyi Mazumdar
Environmental Health.2022;[Epub] CrossRef - Maternal and childhood exposure to inorganic arsenic and airway allergy – A 15-Year birth cohort follow-up study
Tsung-Lin Tsai, Wei-Te Lei, Chin-Chi Kuo, Hai-Lun Sun, Pen-Hua Su, Shu-Li Wang
Environment International.2021; 146: 106243. CrossRef - Joint effect of urinary arsenic species and serum one-carbon metabolism nutrients on gestational diabetes mellitus: A cross-sectional study of Chinese pregnant women
Qiang Zhang, Xumei Zhang, Shuying Li, Huihuan Liu, Liangpo Liu, Qingyu Huang, Yaxing Hou, Xiaoshan Liang, Bo Cui, Ming Zhang, Liting Xia, Liwen Zhang, Chen Li, Jing Li, Guifan Sun, Naijun Tang
Environment International.2021; 156: 106741. CrossRef - Insulin resistance and pancreatic β cell dysfunction are associated with thyroid hormone functions: A cross-sectional hospital-based study in Turkey
Evin Kocatürk, Ezgi Kar, Zeynep Küskü Kiraz, Özkan Alataş
Diabetes & Metabolic Syndrome: Clinical Research & Reviews.2020; 14(6): 2147. CrossRef - Endocrine disruption and obesity: A current review on environmental obesogens
Radhika Gupta, Prashant Kumar, Nighat Fahmi, Bhaskar Garg, Sriparna Dutta, Shilpee Sachar, Avtar S. Matharu, Karani S. Vimaleswaran
Current Research in Green and Sustainable Chemistry.2020; 3: 100009. CrossRef - Circulating miRNA-126, -145 and -155 levels in Mexican women exposed to inorganic arsenic via drinking water
Tania Ruíz-Vera, Ángeles C. Ochoa-Martínez, Sergio Zarazúa, Leticia Carrizales-Yáñez, Iván N. Pérez-Maldonado
Environmental Toxicology and Pharmacology.2019; 67: 79. CrossRef - The role of arsenic in obesity and diabetes
Tahereh Farkhondeh, Saeed Samarghandian, Mohsen Azimi‐Nezhad
Journal of Cellular Physiology.2019; 234(8): 12516. CrossRef - Arsenic and fasting blood glucose in the context of other drinking water chemicals: a cross-sectional study in Bangladesh
Shadassa Ourshalimian, Abu Mohd Naser, Mahbubur Rahman, Solaiman Doza, Jennifer Stowell, K.M. Venkat Narayan, Mohammad Shamsudduha, Matthew O. Gribble
Environmental Research.2019; 172: 249. CrossRef
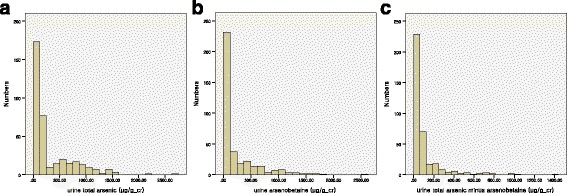
Fig. 1
| Variables | Total | Male | Female | p valuea |
|---|---|---|---|---|
| Number | 369 | 174 | 195 | |
| Age, years | 42.31 ± 15.18b | 41.54 ± 14.62 | 42.99 ± 15.67 | 0.361 |
| Height, cm | 164.06 ± 9.31 | 171.28 ± 6.54 | 157.62 ± 6.14 | <0.001 |
| Weight, kg | 62.83 ± 11.69 | 70.14 ± 10.55 | 56.3 ± 8.32 | <0.001 |
| Waist circumference, cm | 79.73 ± 9.64 | 83.37 ± 9.19 | 76.48 ± 8.85 | <0.001 |
| BMI, kg/cm2 | 23.24 ± 3.22 | 23.9 ± 3.28 | 22.66 ± 3.06 | <0.001 |
| Fasting plasma glucose, mg/dL | 92.35 ± 9.09 | 94.13 ± 9.51 | 90.76 ± 8.4 | <0.001 |
| Blood insulin, mIU/l | 9.28 ± 3.68 | 9.23 ± 4.07 | 9.32 ± 3.31 | 0.828 |
| HOMA-β, % | 122.67 ± 57.85 | 114.92 ± 58.62 | 129.59 ± 56.41 | <0.01 |
| Smoking statusc | <0.001 | |||
| Yes | 104 (28.2%) | 82 (47.1%) | 22 (11.3%) | |
| No | 265 (71.8%) | 92 (52.9%) | 173 (88.7%) | |
| Alcohol consumptiond | <0.001 | |||
| Yes | 224 (60.7%) | 131 (75.3%) | 93 (47.7%) | |
| No | 145 (39.3%) | 43 (24.7%) | 102 (52.3%) | |
| Regular excersicee | 0.065 | |||
| Yes | 106 (28.7%) | 58 (33.3%) | 48 (24.6%) | |
| No | 263 (71.3%) | 116 (66.7%) | 147 (75.4%) | |
| Blood mercury, μg/L | 4.461 (2.982, 5.956)f | 5.465 (3.469, 8.886) | 3.880 (2.782, 5.698) | <0.001 |
| Variables | Total | Male | Female | p valuea |
|---|---|---|---|---|
| urine total arsenic (μg/g_cr) | 136.89 (35.29, 521.39)b | 143.61 (46.97, 563.24) | 123.15 (27.91, 470.27) | 0.055 |
| urine arsenobetaine (μg/g_cr) | 72.13 (16.64, 325.60) | 94.54 (28.46, 369.85) | 51.55 (12.40, 232.58) | <0.05 |
| urine total arsenic minus arsenobetaine (μg/g_cr) | 43.16 (14.53, 106.36) | 46.41 (17.70, 104.80) | 37.83 (13.60, 37.83) | 0.686 |
| Urine arsenobetaine (μg/g_cr) | Total | Male | Female | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Fasting glucose (mg/dL) | p valuea | Post Hocb | Insulin (mIU/L) | p value | Number | Fasting glucose (mg/dL) | p value | Insulin (mIU/L) | p value | Number | Fasting glucose (mg/dL) | p value | Post hoc | Insulin (mIU/L) | p value | |
| 1st quartile (Undetectable-16.36) | 92 | 91.38 ± 8.56c | <0.001 | 1.000 | 9.90 ± 4.04 | 0.259 | 34 | 92.54 ± 8.65 | 0.175 | 10.88 ± 5.20 | 0.055 | 58 | 90.67 ± 8.50 | <0.05 | 0.702 | 9.30 ± 3.04 | 0.947 |
| 2nd quartile (16.91–70.70) | 92 | 89.84 ± 8.22 | Reference | 9.15 ± 3.53 | 47 | 92.56 ± 9.93 | 8.59 ± 3.29 | 45 | 88.24 ± 6.61 | Reference | 9.47 ± 3.66 | ||||||
| 3rd quartile (71.57–321.71) | 92 | 93.39 ± 8.44 | <0.05 | 8.85 ± 3.46 | 45 | 94.03 ± 8.84 | 8.73 ± 3.60 | 47 | 92.29 ± 7.73 | 1.00 | 9.04 ± 3.26 | ||||||
| 4th quartile (324.19–2620.78) | 93 | 94.75 ± 10.31 | <0.001 | 9.21 ± 3.64 | 48 | 96.55 ± 10.39 | 9.08 ± 3.99 | 45 | 92.91 ± 10.02 | <0.05 | 9.34 ± 3.29 | ||||||
| Urine arsenobetaine (μg/g_cr) | Total | Male | Female | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | HOMA-β (%) | p valuea | Post hocb | HOMA-IR | p value | Number | HOMA-β (%) | p value | Post hoc | HOMA-IR | p value | Number | HOMA-β (%) | p value | HOMA-IR | p value | |
| 1st quartile (Undetectable-16.36) | 92 | 135.97 ± 68.01c | <0.01 | Reference | 2.24 ± 1.00 | 0.41 | 35 | 143.74 ± 79.33 | <0.01 | Reference | 2.50 ± 1.27 | 0.146 | 57 | 131.2 ± 60.29 | 0.064 | 2.09 ± 0.76 | 0.966 |
| 2nd quartile (16.91–70.70) | 92 | 132.12 ± 60.6 | 1.000 | 2.04 ± 0.90 | 34 | 112.46 ± 49.85 | 0.146 | 1.99 ± 0.89 | 58 | 143.64 ± 63.7 | 2.08 ± 0.91 | ||||||
| 3rd quartile (71.57–321.71) | 92 | 110.76 ± 48.41 | <0.05 | 2.05 ± 0.97 | 58 | 109.19 ± 53.81 | <0.05 | 2.04 ± 0.90 | 34 | 113.97 ± 38.01 | 2.08 ± 0.87 | ||||||
| 4th quartile (324.19–2620.78) | 93 | 111.76 ± 48.34 | <0.05 | 2.18 ± 0.94 | 47 | 102.29 ± 45.38 | <0.01 | 2.16 ± 1.04 | 46 | 121.44 ± 49.84 | 2.15 ± 0.85 | ||||||
| Variables | Total | Male | Female | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HOMA-β (%) | HOMA-IR | HOMA-β (%) | HOMA-IR | HOMA-β (%) | HOMA-IR | |||||||
| Coefficient (95% C.I) | p value | Coefficient (95% C.I) | p value | Coefficient (95% C.I) | p value | Coefficient (95% C.I) | p value | Coefficient (95% C.I) | p-value | Coefficient (95% C.I) | p value | |
| Urine arsenobetaine (μg/g_cr) | ||||||||||||
| 1st quartile (Undetectable-16.36) | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| 2nd quartile (16.91–70.70) | −9.613 (−24.490, 5.264) | 0.205 | −0.208(−0.449, 0.033) | 0.091 | −30.043(−53.954, −6.133) | <0.05 | −0.405(−0.835, −0.025) | 0.065 | 5.394(−13.276, 24.064) | 0.571 | −0.059(−0.329, 0.211) | 0.668 |
| 3rd quartile (71.57–321.71) | −17.796(−33.257, −2.334) | <0.05 | −0.219(−0.469, 0.031) | 0.086 | −22.382(−44.023, −0.742) | <0.05 | −0.343(−0.732, 0.046) | 0.084 | −12.575(−35.104, 9.954) | 0.274 | −0.071(−0.397, 0.254) | 0.667 |
| 4th quartile (324.19–2620.78) | −8.368(−24.891, 8.056) | 0.318 | −0.038(−0.304,0.228) | 0.779 | −14.950(−39.251, 9.351) | 0.228 | −0.027(−0.464, 0.410) | 0.902 | −3.857(−25.647, 17.932) | 0.729 | −0.055(−0.370, 0.259) | 0.731 |
| Age (years) | 01.117(−1.486,0.748) | <0.001 | −0.004(−0.010, 0.002) | 0.172 | −1.211(−1.752, −0.671) | <0.001 | −0.006(−0.015, 0.004) | 0.258 | −1.052(−1.558, −0.546) | <0.001 | −0.003(−0.011, 0.004) | 0.376 |
| BMI (kg/cm2) | 5.188 (3.523, 6.853) | <0.001 | 0.136 (0.109, 0.163) | <0.001 | 5.465 (3.075,7.855) | <0.001 | 0.149 (0.106, 0.192) | <0.001 | 5.565 (3.184, 7.946) | <0.001 | 0.132 (0.097, 0.166) | <0.001 |
| Smokinga | −10.452(−22.42, 1.515) | 0.087 | −0.114(−0.308, 0.080) | 0.248 | −11.827(−27.403,3.750) | 0.137 | −0.251(−0.531, 0.029) | 0.079 | 8.524 (−14.362, 31.410) | 0.465 | 0.242(−0.088, 0.572) | 0.151 |
| Alcohol consumptionb | −14.864(−26.291, −3.436) | <0.05 | 0.065(−0.120, 0.250) | 0.492 | −18.557(−37.121, 0.006) | 0.050 | 0.077(−0.256, 0.411) | 0.650 | −9.699(−24.618, 5.219) | 0.203 | 0.045(−0.170, 0.261) | 0.680 |
| Regular exercisec | −4.067(−15.791, 7.657) | 0.497 | −0.137(−0.327, 0.053) | 0.157 | −2.490(−18.472, 13.492) | 0.760 | −0.031(−0.318, 0.256) | 0.833 | −4.731(−21.725, 12.264) | 0.585 | −0.260(−0.505, −0.015) | <0.05 |
| Blood mercury (μg/L) | ||||||||||||
| 1st quartile (1.188–2.977) | Reference | Reference | Reference | Reference | Reference | Reference | ||||||
| 2nd quartile (2.987–4.598) | 15.686 (1.036,30.337) | <0.05 | −0.046(−0.283, 0.191) | 0.705 | −0.415(−25.210, 24.381) | 0.974 | −0.264(−0.710, 0.182) | 0.246 | 27.067 (8.938, 45.197) | <0.01 | 0.108(−0.153, 0.370) | 0.417 |
| 3rd quartile (4.625–6.969) | 5.642(−10.252, 21.536) | 0.487 | 0.023(−0.235, 0.280) | 0.862 | −2.752(−27.702, 22.197) | 0.829 | −0.388(−0.836, 0.061) | 0.090 | 11.404(−9.568, 32.377) | 0.287 | 0.304 (0.001, 0.607) | <0.05 |
| 4th quartile (6.997–37.654) | −15.172(−32.179, 1.835) | 0.08 | −0.165(−0.440, 0.110) | 0.240 | −27.387(−52.474, −2.300) | <0.05 | −0.524(−0.975, −0.073) | <0.05 | 3.667(−21.177, 28.512) | 0.772 | 0.178(−0.181, 0.536) | 0.332 |
a
bValues are presented as arithmetic mean ± standard deviation
cSmoking status was indicated as ‘yes’ for participants who had smoked more than five packs of cigarettes during their life and were currently smoking
dAlcohol consumption was indicated as ‘yes’ for participants who consumed at least one glass of alcohol every month over the previous year
eRegular exercise was indicated as ‘yes’ when the participant performed mederate or strenuous exercise on a regular basis (for more than 30 min at a time and more than five times per week in the case of moderate exercise; for more than 20 min at a time in the case of strenuous exercise)
fValues are presented as median (25th percentile, 75th percentile)
a
bValues are presented as median (25th percentile, 75th percentile)
a
bPost hoc by Bonferroni test
cValues are presented as arithmetic mean ± standard deviation
a
bPost hoc by Bonferroni test
cValues are presented as arithmetic mean ± standard deviation
aSmoking status was indicated as ‘yes’ for participants who had smoked more than five packs of cigarettes during their life and were currently smoking
bAlcohol consumption was indicated as ‘yes’ for participants who consumed at least one glass of alcohol every month over the previous year
cRegular exercise was indicated as ‘yes’ when the participant performed mederate or strenuous exercise on a regular basis (for more than 30 min at a time and more than five times per week in the case of moderate exercise; for more than 20 min at a time in the case of strenuous exercise)
 KSOEM
KSOEM

 Cite
Cite

