Articles
- Page Path
- HOME > Ann Occup Environ Med > Volume 34; 2022 > Article
- Original Article Correlation between shift work and non-alcoholic fatty liver disease among male workers in the steel manufacturing company of Korea: a cross-sectional study
-
Kiseok Kim
 , Yong-Jin Lee
, Yong-Jin Lee , Soon-Chan Kwon
, Soon-Chan Kwon , Young-Sun Min
, Young-Sun Min , Hyun Kyo Lee
, Hyun Kyo Lee , Gwangin Baek
, Gwangin Baek , Sang Hyeon Kim
, Sang Hyeon Kim , Eun-Chul Jang
, Eun-Chul Jang
-
Annals of Occupational and Environmental Medicine 2022;34:e33.
DOI: https://doi.org/10.35371/aoem.2022.34.e33
Published online: November 2, 2022
Department of Occupational and Environmental Medicine, Soonchunhyang University Cheonan Hospital, Cheonan, Korea.
- Correspondence: Eun-Chul Jang. Department of Occupational and Environmental Medicine, Soonchunhyang University Cheonan Hospital, 31 Suncheonhyang 6-gil, Dongnam-gu, Cheonan 31151, Korea. oemdr10@gmail.com
Copyright © 2022 Korean Society of Occupational & Environmental Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background Circadian rhythm disturbance caused by shift work has adverse effects on the metabolic homeostasis of the liver. Disruption of the metabolic homeostasis of the liver causes fat accumulation in the liver. The aim of this study was to investigate the correlation between shift work and non-alcoholic fatty liver disease (NAFLD) among male workers in the steel manufacturing industry of Korea.
-
Methods Based on medical examination data collected in June 2020, 2,511 male subjects from one steel manufacturing company in Korea were selected in total. NAFLD was evaluated using abdominal ultrasound, which was performed by two experienced radiologists. The multinomial logistic regression analysis was performed by adjusting for age, physical activity, smoking history, alcohol consumption, body mass index, waist circumference, blood pressure, blood glucose, lipidemia, liver function test, employment duration, and hepatotoxic materials exposure status.
-
Results Compared to daytime workers, the odds ratio (OR) of moderate-severe NAFLD in shift workers was 1.449 (95% confidence interval [CI], 1.028–2.043). Compared to daytime workers, the ORs of moderate-severe NAFLD were significantly higher for the group that engaged in total shift work for more than 20 years (OR, 2.285; 95% CI, 1.051–4.970), the group that was not allowed to sleep during night shift work (OR, 1.463; 95% CI, 1.030–2.078), and the group that consumed food during night shift work (OR, 1.580; 95% CI, 1.093–2.284).
-
Conclusions There was a correlation between shift work and moderate-severe NAFLD in male steel manufacturing workers. There will be a need for more research related to the correlation of shift work with steatohepatitis and cirrhosis in the future.
BACKGROUND
METHODS
RESULTS
General characteristics of study subjects
Shift work-related variables of study subjects
OR of NAFLD according to shift work
ORs of NAFLD according to shift work-related variables
DISCUSSION
CONCLUSIONS
ACKNOWLEDGEMENTS
-
Funding: This work was supported by the Soonchunhyang University Research Fund.
-
Competing interests: The authors declare that they have no competing interests.
-
Author Contributions:
NOTES
- 1. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67(1):328–357. 28714183.ArticlePubMedPDF
- 2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64(1):73–84. 26707365.ArticlePubMed
- 3. Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, et al. 2019 Global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2021.
- 4. Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2019;4(5):389–398. 30902670.ArticlePubMed
- 5. Im HJ, Ahn YC, Wang JH, Lee MM, Son CG. Systematic review on the prevalence of nonalcoholic fatty liver disease in South Korea. Clin Res Hepatol Gastroenterol 2021;45(4):101526. 32919911.ArticlePubMed
- 6. Paik JM, Henry L, De Avila L, Younossi E, Racila A, Younossi ZM. Mortality related to nonalcoholic fatty liver disease is increasing in the United States. Hepatol Commun 2019;3(11):1459–1471. 31701070.ArticlePubMedPMCPDF
- 7. Ahmed A, Wong RJ, Harrison SA. Nonalcoholic fatty liver disease review: diagnosis, treatment, and outcomes. Clin Gastroenterol Hepatol 2015;13(12):2062–2070. 26226097.ArticlePubMed
- 8. Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015;62(6):1723–1730. 26274335.ArticlePubMedPDF
- 9. Wickwire EM, Geiger-Brown J, Scharf SM, Drake CL. Shift work and shift work sleep disorder: clinical and organizational perspectives. Chest 2017;151(5):1156–1172. 28012806.ArticlePubMedPMC
- 10. Manohar S, Thongprayoon C, Cheungpasitporn W, Mao MA, Herrmann SM. Associations of rotational shift work and night shift status with hypertension: a systematic review and meta-analysis. J Hypertens 2017;35(10):1929–1937. 28650914.PubMed
- 11. Gan Y, Yang C, Tong X, Sun H, Cong Y, Yin X, et al. Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup Environ Med 2015;72(1):72–78. 25030030.ArticlePubMed
- 12. Dutheil F, Baker JS, Mermillod M, De Cesare M, Vidal A, Moustafa F, et al. Shift work, and particularly permanent night shifts, promote dyslipidaemia: a systematic review and meta-analysis. Atherosclerosis 2020;313:156–169. 33069952.ArticlePubMed
- 13. Puttonen S, Härmä M, Hublin C. Shift work and cardiovascular disease - pathways from circadian stress to morbidity. Scand J Work Environ Health 2010;36(2):96–108. 20087536.ArticlePubMed
- 14. Saran AR, Dave S, Zarrinpar A. Circadian rhythms in the pathogenesis and treatment of fatty liver disease. Gastroenterology 2020;158(7):1948–1966.e1. 32061597.ArticlePubMed
- 15. Zhang S, Wang Y, Wang Z, Wang H, Xue C, Li Q, et al. Rotating night shift work and non-alcoholic fatty liver disease among steelworkers in China: a cross-sectional survey. Occup Environ Med 2020;77(5):333–339. 32019846.ArticlePubMed
- 16. Choi H, Oh HJ, Shin JS, Lim M, Kim SK, Kang HT, et al. Relationship between shift work and liver enzymes: a cross-sectional study based on the Korea National Health and Examination Survey (2007–2015). Ann Occup Environ Med 2019;31(1):e15. 31583106.PubMedPMC
- 17. Kang SH, Lee HW, Yoo JJ, Cho Y, Kim SU, Lee TH, et al. KASL clinical practice guidelines: management of nonalcoholic fatty liver disease. Clin Mol Hepatol 2021;27(3):363–401. 34154309.ArticlePubMedPMCPDF
- 18. Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54(24):1451–1462. 33239350.ArticlePubMedPMC
- 19. Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract 2007;75(1):72–80. 16735075.ArticlePubMed
- 20. Seo MH, Lee WY, Kim SS, Kang JH, Kang JH, Kim KK, et al. 2018 Korean society for the study of obesity guideline for the management of obesity in Korea. J Obes Metab Syndr 2019;28(1):40–45. 31089578.PubMedPMC
- 21. Lee HY, Shin J, Kim GH, Park S, Ihm SH, Kim HC, et al. 2018 Korean Society of Hypertension guidelines for the management of hypertension: part II-diagnosis and treatment of hypertension. Clin Hypertens 2019;25(1):20. 31388453.ArticlePubMedPMCPDF
- 22. Hur KY, Moon MK, Park JS, Kim SK, Lee SH, Yun JS, et al. 2021 clinical practice guidelines for diabetes mellitus of the Korean Diabetes Association. Diabetes Metab J 2021;45(4):461–481. 34352984.PubMedPMC
- 23. Rhee EJ, Kim HC, Kim JH, Lee EY, Kim BJ, Kim EM, et al. 2018 guidelines for the management of dyslipidemia in Korea. J Lipid Atheroscler 2019;8(2):78–131. 32821702.ArticlePubMedPMCPDF
- 24. Ministry of Employment and Labor. Enforcement Regulations of Occupational Safety and Health Act. Updated 2022]. Accessed July 25, 2022]. https://www.law.go.kr/LSW//admRulBylInfoPLinkR.do?admRulSeq=2100000212249&admRulNm=%EA%B1%B4%EA%B0%95%EA%B2%80%EC%A7%84%20%EC%8B%A4%EC%8B%9C%EA%B8%B0%EC%A4%80&bylNo=0004&bylBrNo=00&bylCls=BE&bylClsCd=BE&joEfYd=&bylEfYd= .
- 25. Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines: management of alcoholic liver disease. Clin Mol Hepatol 2013;19(3):216–254. 24133661.ArticlePubMedPMC
- 26. Lee S, Kim JS, Jung JG, Oh MK, Chung TH, Kim J. Korean alcohol guidelines for moderate drinking based on facial flushing. Korean J Fam Med 2019;40(4):204–211. 31302995.ArticlePubMedPMCPDF
- 27. Ministry of Employment and Labor. Enforcement Regulations of Occupational Safety and Health Act. Updated 2021]. Accessed April 5, 2022]. https://www.law.go.kr/LSW//lsBylInfoPLinkR.do?lsiSeq=212709&lsNm=%EC%82%B0%EC%97%85%EC%95%88%EC%A0%84%EB%B3%B4%EA%B1%B4%EB%B2%95+%EC%8B%9C%ED%96%89%EA%B7%9C%EC%B9%99&bylNo=0022&bylBrNo=00&bylCls=BE&bylEfYd=20200116&bylEfYdYn=Y .
- 28. Wahlang B, Beier JI, Clair HB, Bellis-Jones HJ, Falkner KC, McClain CJ, et al. Toxicant-associated steatohepatitis. Toxicol Pathol 2013;41(2):343–360. 23262638.ArticlePubMedPMCPDF
- 29. Korean Industrial Health Association. Updated 2018]. Accessed July 25, 2022]. https://kiha21.or.kr/doc_download/night_ko_2018.pdf .
- 30. Ferraioli G, Soares Monteiro LB. Ultrasound-based techniques for the diagnosis of liver steatosis. World J Gastroenterol 2019;25(40):6053–6062. 31686762.ArticlePubMedPMC
- 31. Younossi ZM. Non-alcoholic fatty liver disease - a global public health perspective. J Hepatol 2019;70(3):531–544. 30414863.ArticlePubMed
- 32. Wu KT, Kuo PL, Su SB, Chen YY, Yeh ML, Huang CI, et al. Nonalcoholic fatty liver disease severity is associated with the ratios of total cholesterol and triglycerides to high-density lipoprotein cholesterol. J Clin Lipidol 2016;10(2):420–5.e1. 27055973.ArticlePubMed
- 33. Lonardo A, Bellentani S, Argo CK, Ballestri S, Byrne CD, Caldwell SH, et al. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig Liver Dis 2015;47(12):997–1006. 26454786.ArticlePubMed
- 34. Hong HC, Hwang SY, Choi HY, Yoo HJ, Seo JA, Kim SG, et al. Relationship between sarcopenia and nonalcoholic fatty liver disease: the Korean Sarcopenic Obesity Study. Hepatology 2014;59(5):1772–1778. 23996808.ArticlePubMed
- 35. Chung GE, Lee Y, Yim JY, Choe EK, Kwak MS, Yang JI, et al. Genetic polymorphisms of PNPLA3 and SAMM50 are associated with nonalcoholic fatty liver disease in a Korean population. Gut Liver 2018;12(3):316–323. 29271184.ArticlePubMedPMC
- 36. Guo Z, Li M, Han B, Qi X. Association of non-alcoholic fatty liver disease with thyroid function: a systematic review and meta-analysis. Dig Liver Dis 2018;50(11):1153–1162. 30224316.ArticlePubMed
- 37. Adams LA, Feldstein A, Lindor KD, Angulo P. Nonalcoholic fatty liver disease among patients with hypothalamic and pituitary dysfunction. Hepatology 2004;39(4):909–914. 15057893.ArticlePubMed
- 38. Chung JH. Evaluation of thyroid hormone levels and urinary iodine concentrations in Koreans based on the data from Korea National Health and Nutrition Examination Survey VI (2013 to 2015). Endocrinol Metab (Seoul) 2018;33(2):160–163. 29766681.ArticlePubMedPMCPDF
- 39. Kim SY. Diagnosis and treatment of hypopituitarism. Endocrinol Metab (Seoul) 2015;30(4):443–455. 26790380.ArticlePubMedPMC
- 40. Zisapel N. Circadian rhythm sleep disorders: pathophysiology and potential approaches to management. CNS Drugs 2001;15(4):311–328. 11463135.ArticlePubMed
- 41. Mukherji A, Bailey SM, Staels B, Baumert TF. The circadian clock and liver function in health and disease. J Hepatol 2019;71(1):200–211. 30930223.ArticlePubMedPMC
- 42. Wei T, Li C, Heng Y, Gao X, Zhang G, Wang H, et al. Association between night-shift work and level of melatonin: systematic review and meta-analysis. Sleep Med 2020;75:502–509. 33022488.ArticlePubMed
- 43. Wei Y, Rector RS, Thyfault JP, Ibdah JA. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J Gastroenterol 2008;14(2):193–199. 18186554.ArticlePubMedPMC
- 44. Solís-Muñoz P, Solís-Herruzo JA, Fernández-Moreira D, Gómez-Izquierdo E, García-Consuegra I, Muñoz-Yagüe T, et al. Melatonin improves mitochondrial respiratory chain activity and liver morphology in ob/ob mice. J Pineal Res 2011;51(1):113–123. 21355880.ArticlePubMed
- 45. Lee SI, Nishi T, Takahashi M, Higuchi S. Effects of 2-hour nighttime nap on melatonin concentration and alertness during 12-hour simulated night work. Ind Health 2021;59(6):393–402. 34588379.ArticlePubMedPMC
- 46. Lowden A, Moreno C, Holmbäck U, Lennernäs M, Tucker P. Eating and shift work - effects on habits, metabolism and performance. Scand J Work Environ Health 2010;36(2):150–162. 20143038.ArticlePubMed
- 47. Hampton SM, Morgan LM, Lawrence N, Anastasiadou T, Norris F, Deacon S, et al. Postprandial hormone and metabolic responses in simulated shift work. J Endocrinol 1996;151(2):259–267. 8958786.ArticlePubMed
- 48. Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology 2005;42(5):987–1000. 16250043.ArticlePubMed
- 49. Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med 2011;43(8):617–649. 21039302.ArticlePubMed
- 50. Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2015;149(2):389–397.e10. 25935633.ArticlePubMed
REFERENCES
REFERENCES
Figure & Data
REFERENCES
Citations

- Rythmes biologiques et métabolisme
A. Hebras, M. Adlanmerini, H. Duez
EMC - Endocrinologie - Nutrition.2025; 36(1): 1. CrossRef - Prevalence of metabolic dysfunction-associated fatty liver disease among information technology employees in India
Bharam Bhargava, Padaki Nagaraja Rao, Anand V. Kulkarni, Ravikanth Vishnubhotla, Nanditha Pramod, Chandanadur Thippaiah Anitha, Kalyankar Mahadev
Scientific Reports.2025;[Epub] CrossRef - The relationship between shift work and steatotic liver disease associated with metabolic dysfunction (MASLD): a systematic scoping review
Virgínia S. D. Oliveira, Sara Montagnese, Claudia R. C. Moreno
Journal of Public Health.2025;[Epub] CrossRef - Shift work and metabolic dysfunction-associated steatotic liver disease: a systematic review of observational studies
Bingya Ma, Yihang Fan, Wenjun Fan
International Archives of Occupational and Environmental Health.2025;[Epub] CrossRef - Nonalcoholic Fatty Liver Disease in Shift Workers and Its Effect on Peripheral Nerve Conduction: A Cross-Sectional Study
Dipali K Chatur, Saroj K Pati, Jayshri R Ghate, Rachita Nanda, Meenakshi Sinha, Kalpana Kodapi
Cureus.2024;[Epub] CrossRef - Shift work promotes adipogenesis via cortisol-dependent downregulation of EGR3-HDAC6 pathway
Xinxing Wan, Linghao Wang, Md Asaduzzaman Khan, Lin Peng, Keke Zhang, Xiaoying Sun, Xuan Yi, Zhouqi Wang, Ke Chen
Cell Death Discovery.2024;[Epub] CrossRef - Inter-individual variations in circadian misalignment-induced NAFLD pathophysiology in mice
Nobuya Koike, Yasuhiro Umemura, Hitoshi Inokawa, Isao Tokuda, Yoshiki Tsuchiya, Yuh Sasawaki, Atsushi Umemura, Naoko Masuzawa, Kazuya Yabumoto, Takashi Seya, Akira Sugimoto, Seung-Hee Yoo, Zheng Chen, Kazuhiro Yagita
iScience.2024; 27(2): 108934. CrossRef - Effects of Oat β-Glucan and Inulin on Alleviation of Nonalcoholic Steatohepatitis Aggravated by Circadian Disruption in C57BL/6J Mice
Nelson Kei, Kam Kuen Cheung, Ka Lee Ma, Tsz Kwan Yau, Susana Lauw, Vincent Wai Sun Wong, Lijun You, Peter Chi Keung Cheung
Journal of Agricultural and Food Chemistry.2024; 72(7): 3520. CrossRef - Circadian Deregulation: Back Facing the Sun Toward Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Development
Mariana Verdelho Machado
Nutrients.2024; 16(24): 4294. CrossRef - Night shift-induced circadian disruption: links to initiation of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and risk of hepatic cancer
Anjali Singh, Baby Anjum, Qulsoom Naz, Sana Raza, Rohit A. Sinha, Mohammad Kaleem Ahmad, Abbas Ali Mehdi, Narsingh Verma
Hepatoma Research.2024;[Epub] CrossRef - Assessing Dust Emissions, Health Impacts, and Accident Risks in Prefabricated and Conventional Construction: A Comprehensive Comparative Study
Louis Kumi, Jaewook Jeong, Jaemin Jeong
Buildings.2023; 13(9): 2305. CrossRef
- Figure
- Related articles
-
- The association of shift work and TyG index among male workers in a chemical plant of Korea: a cross-sectional study
- Association between serum perfluoroalkyl substances concentrations and non-alcoholic fatty liver disease among Korean adults: a cross-sectional study using the National Environmental Health Survey cycle 4
- Association between shift work and the risk of hypothyroidism in adult male workers in Korea: a cohort study
- Relationship between job stress and impaired fasting glucose in male steel industry workers: a cross-sectional study
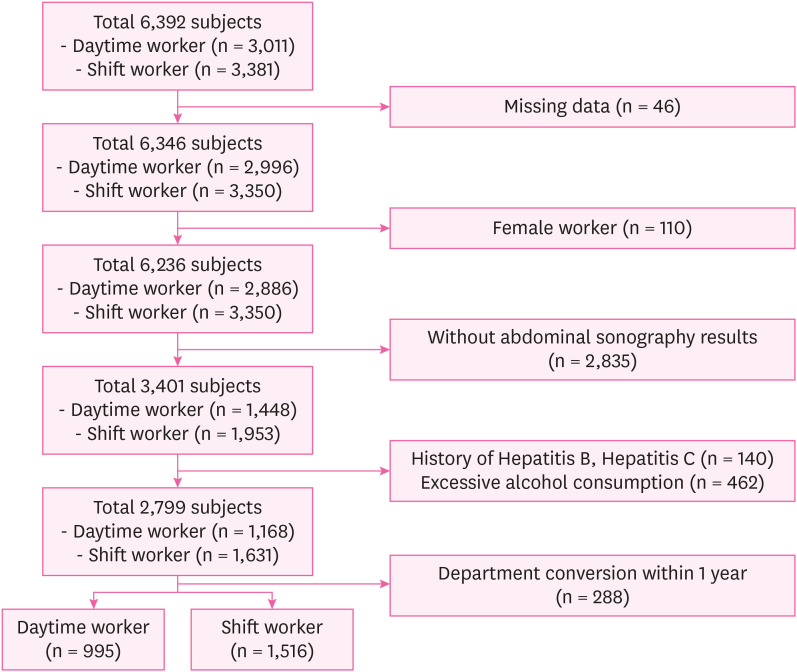
Fig. 1
| Variables | Total subjects (n = 2,511) | Daytime worker (n = 995) | Shift worker (n = 1,516) | ||
|---|---|---|---|---|---|
| Age (yr) | 36.97 ± 8.46 | 37.62 ± 9.13 | 36.54 ± 7.97 | 0.002a,c | |
| Age group | 0.015b,d | ||||
| < 30 yr | 374 (14.9) | 150 (15.1) | 224 (14.8) | ||
| 30–39 yr | 1,334 (53.1) | 506 (50.8) | 828 (54.6) | ||
| 40–49 yr | 512 (20.4) | 199 (20.0) | 313 (20.6) | ||
| ≥ 50 yr | 291 (11.6) | 140 (14.1) | 151 (10.0) | ||
| Duration of employment (mon) | 117.90 ± 73.68 | 110.66 ± 77.22 | 122.66 ± 70.88 | < 0.001a,e | |
| Duration of employment group | 0.729b | ||||
| < 10 yr | 1,612 (64.2) | 648 (65.1) | 964 (63.6) | ||
| 10–19 yr | 721 (28.7) | 279 (28.1) | 442 (29.1) | ||
| ≥ 20 yr | 178 (7.1) | 68 (6.8) | 110 (7.3) | ||
| NAFLD | 0.002b,c | ||||
| Normal | 1,393 (55.5) | 552 (55.5) | 841 (55.5) | ||
| Light grade | 716 (28.5) | 312 (31.4) | 404 (26.6) | ||
| Moderate-severe grade | 402 (16.0) | 131 (13.1) | 271 (17.9) | ||
| Alcohol consumption | 0.087b | ||||
| Non drinker | 351 (14.0) | 121 (12.2) | 230 (15.2) | ||
| Moderate drinker | 1,814 (72.2) | 729 (73.3) | 1,085 (71.6) | ||
| Heavy drinker | 346 (13.8) | 145 (14.5) | 201 (13.2) | ||
| Smoking | 0.034b,d | ||||
| Non-smoker | 1,117 (44.5) | 469 (47.1) | 648 (42.7) | ||
| Ex-smoker | 771 (30.7) | 304 (30.6) | 467 (30.8) | ||
| Current smoker | 623 (24.8) | 222 (22.3) | 401 (26.5) | ||
| Physical activity | 0.875b | ||||
| Health promotion | 1,073 (42.7) | 419 (39.1) | 654 (43.1) | ||
| Recommended | 511 (20.4) | 204 (20.5) | 307 (20.3) | ||
| Danger | 927 (36.9) | 372 (37.4) | 555 (36.6) | ||
| BMI | 0.144b | ||||
| Normal | 465 (18.5) | 177 (17.8) | 288 (19.0) | ||
| Pre-obese | 678 (27.0) | 255 (25.7) | 423 (27.9) | ||
| Obese class 1 | 1,128 (44.9) | 475 (47.7) | 653 (43.1) | ||
| Obese class 2 | 240 (9.6) | 88 (8.8) | 152 (10.0) | ||
| Waist circumference | 0.837b | ||||
| Normal | 1,829 (72.8) | 727 (73.1) | 1,102 (72.7) | ||
| Abdominal obesity | 682 (27.2) | 268 (26.9) | 414 (27.3) | ||
| Abnormal blood pressure | 0.136b | ||||
| No | 2,261 (90.0) | 885 (88.9) | 1,376 (90.8) | ||
| Yes | 250 (10.0) | 110 (11.1) | 140 (9.2) | ||
| Abnormal blood glucose | 0.192b | ||||
| No | 2,434 (96.9) | 970 (97.5) | 1,464 (96.6) | ||
| Yes | 77 (3.1) | 25 (2.5) | 52 (3.4) | ||
| Dyslipidemia | 0.764b | ||||
| No | 1,624 (64.7) | 640 (64.3) | 984 (64.9) | ||
| Yes | 887 (35.3) | 355 (35.7) | 532 (35.1) | ||
| Abnormal liver enzyme | 0.021b,d | ||||
| No | 1,746 (69.5) | 718 (72.2) | 1,028 (67.8) | ||
| Yes | 765 (30.5) | 277 (27.8) | 488 (32.2) | ||
| TASH-causing materials | < 0.001b,e | ||||
| No | 1,171 (46.6) | 600 (60.3) | 571 (37.7) | ||
| Yes | 1,340 (53.4) | 395 (39.7) | 945 (62.3) | ||
| Variables | Total (n = 1,516) | Normal (n = 841) | Light NAFLD (n = 404) | Moderate-Severe NAFLD (n = 271) | ||
|---|---|---|---|---|---|---|
| Total duration of shift work | 0.005a,b | |||||
| < 10 yr | 890 (58.7) | 529 (59.4) | 216 (24.3) | 145 (16.3) | ||
| 10–19 yr | 459 (30.3) | 234 (51.0) | 137 (29.8) | 88 (19.2) | ||
| ≥ 20 yr | 167 (11.0) | 78 (46.7) | 51 (30.5) | 38 (22.8) | ||
| Sleeping during night shift work | 0.085a | |||||
| Allowed | 176 (11.6) | 109 (61.9) | 35 (19.9) | 32 (18.2) | ||
| Not allowed | 1,340 (88.4) | 732 (54.6) | 369 (27.5) | 239 (17.8) | ||
| Consuming food during night shift work | 0.023a,c | |||||
| No | 490 (32.3) | 260 (53.1) | 152 (31.0) | 78 (15.9) | ||
| Yes | 1,026 (67.7) | 581 (56.6) | 252 (24.6) | 193 (18.8) | ||
| Variables | Total subjects | Light NAFLD | Moderate-severe NAFLD | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||||
| Model 1a | ||||||
| Daytime work | 995 (39.6) | Reference | Reference | |||
| Shift work | 1,516 (60.4) | 0.850 (0.708–1.020) | 0.081 | 1.358 (1.074–1.717) | 0.011e | |
| Model 2b | ||||||
| Daytime work | 995 (39.6) | Reference | Reference | |||
| Shift work | 1,516 (60.4) | 0.841 (0.699–1.013) | 0.069 | 1.306 (1.027–1.659) | 0.029e | |
| Model 3c | ||||||
| Daytime work | 995 (39.6) | Reference | Reference | |||
| Shift work | 1,516 (60.4) | 0.874 (0.699–1.094) | 0.239 | 1.449 (1.028–2.043) | 0.034e | |
| Variables | Total subjects | Model 1a | Model 2b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Light NAFLD | Moderate-severe NAFLD | Light NAFLD | Moderate-Severe NAFLD | |||||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |||||||
| Total duration of shift work | ||||||||||
| Daytime work | 995 (39.6) | Reference | Reference | Reference | Reference | |||||
| < 10 yr | 890 (35.4) | 0.722 (0.585–0.892) | 0.002d | 1.155 (0.886–1.505) | 0.286 | 0.830 (0.631–1.092) | 0.183 | 1.322 (0.873–2.004) | 0.188 | |
| 10–19 yr | 459 (18.3) | 1.036 (0.805–1.333) | 0.785 | 1.585 (1.162–2.161) | 0.004d | 0.956 (0.689–1.327) | 0.789 | 1.409 (0.869–2.287) | 0.164 | |
| ≥ 20 yr | 167 (6.7) | 1.157 (0.792–1.690) | 0.452 | 2.053 (1.333–3.162) | 0.001d | 0.889 (0.520–1.521) | 0.667 | 2.285 (1.051–4.970) | 0.037e | |
| Sleeping during night shift work | ||||||||||
| Daytime work | 995 (39.6) | Reference | Reference | Reference | Reference | |||||
| Allowed | 176 (7.0) | 0.568 (0.379–0.852) | 0.006d | 1.237 (0.799–1.916) | 0.341 | 0.606 (0.376–0.978) | 0.040e | 1.355 (0.704–2.607) | 0.364 | |
| Not allowed | 1,340 (53.4) | 0.892 (0.740–1.075) | 0.230 | 1.376 (1.082–1.749) | 0.009d | 0.913 (0.726–1.148) | 0.437 | 1.463 (1.030–2.078) | 0.034e | |
| Consuming food during night shift work | ||||||||||
| Daytime work | 995 (39.6) | Reference | Reference | Reference | Reference | |||||
| No | 490 (19.5) | 1.034 (0.811–1.320) | 0.786 | 1.264 (0.921–1.735) | 0.147 | 0.963 (0.717–1.295) | 0.805 | 1.186 (0.751–1.873) | 0.464 | |
| Yes | 1,026 (40.9) | 0.767 (0.627–0.940) | 0.010e | 1.400 (1.089–1.798) | 0.009d | 0.831 (0.650–1.062) | 0.139 | 1.580 (1.093–2.284) | 0.015e | |
Categorical variables are presented as number (%). Continuous variables are presented as mean ± standard deviation.
NAFLD: non-alcoholic fatty liver disease; BMI: body mass index; TASH: toxic-associated steatohepatitis.
a
Variables are presented as number (%).
NAFLD: non-alcoholic fatty liver disease.
a
OR: odds ratio; NAFLD: non-alcoholic fatty liver disease; CI: confidence interval.
aModel 1: unadjusted; bModel 2: adjusted for age, alcohol consumption, smoking, physical activity; cModel 3: adjusted for age, alcohol consumption, smoking, physical activity, body mass index, waist circumference, duration of employment, blood pressure, blood glucose, lipidemia, liver function test, toxic-associated steatohepatitis -causing materials; d
aModel 1: unadjusted; bModel 2: adjusted for age, alcohol consumption, smoking, physical activity, body mass index, waist circumference, duration of employment, blood pressure, blood glucose, lipidemia, liver function test, toxic-associated steatohepatitis-causing materials; c
OR: odds ratio; NAFLD: non-alcoholic fatty liver disease; CI: confidence interval.
 KSOEM
KSOEM

 Cite
Cite

