Articles
- Page Path
- HOME > Ann Occup Environ Med > Volume 34; 2022 > Article
- Case Report A case report of toxic hepatitis caused by chloroform in automotive parts manufacturer coating process
-
Jong Hyun Hwang1
 , Jung Il Kim2
, Jung Il Kim2
-
Annals of Occupational and Environmental Medicine 2022;34:e22.
DOI: https://doi.org/10.35371/aoem.2022.34.e22
Published online: August 29, 2022
1Department of Occupational and Environmental Medicine, Dong-A University Hospital, Busan, Korea.
2Department of Occupational and Environmental Medicine, College of Medicine, Dong-A University, Busan, Korea.
- Correspondence: Jung Il Kim. Department of Occupational and Environmental Medicine, College of Medicine, Dong-A University, 32 Daesingongwon-ro, Seo-gu, Busan 49201, Korea. kimji@dau.ac.kr
Copyright © 2022 Korean Society of Occupational & Environmental Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background Several cases of chloroform-induced hepatotoxicity have been reported worldwide, but only 2 cases have been reported in Korea. We encountered a case of toxic hepatitis due to chloroform exposure in February 2022 and report the diagnosis process and clinical findings.
-
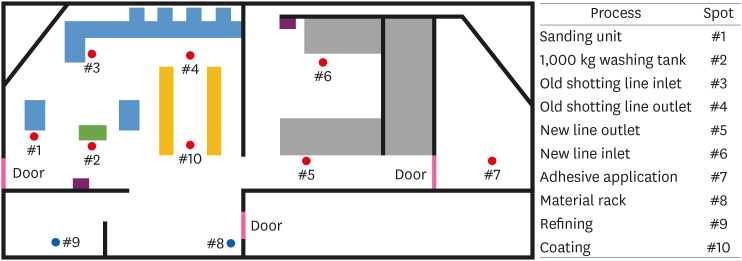
Case presentation A 38-year-old employee in charge of the coating after washing (degreasing) at an automotive parts manufacturer complained of jaundice and was diagnosed with acute toxic hepatitis. After the initial diagnosis, he continued to work, his symptoms worsened, and he was hospitalized for 8 days. Liver ultrasonography (elastography) revealed acute hepatitis. The washing agent contained chloroform, which was not listed on the materials safety data sheet, and the concentrations of chloroform in the workplace were up to 4.7 times the time-weighted average.
-
Conclusions This patient showed typical toxic hepatitis with chloroform; further follow-up studies are required. Both employers and workers should be aware of information on toxic substances and take precautions to avoid exposure.
BACKGROUND
CASE PRESENTATION
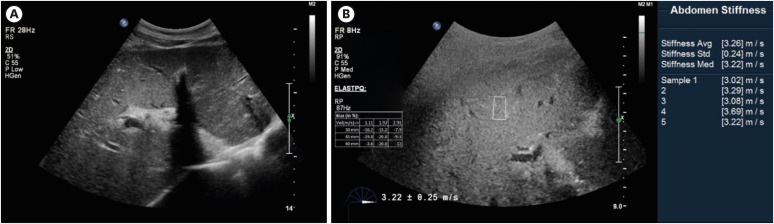
Abdominal ultrasonography. (A) The liver shows diffuse decreased parenchymal echogenicity with prominent portal venular wall echogenicity. The gallbladder shows diffuse wall edema. (B) Elastography: the mean shear wave velocity was 3.26 ± 0.24 m/s.
Summary of biochemical analysis of patient by date
DISCUSSION AND CONCLUSION
Occupational liver diseases reported in the Annals of Occupational and Environmental Medicine
| Exposed chemicals | Year | Number of patients | Diagnosis | Ultrasonography | Elastography | CT | Biopsy |
|---|---|---|---|---|---|---|---|
| Trichloroethylene13 | 1999 | 1 | Toxic hepatitis, exfoliative dermatitis | Enhanced intrahepatic echo | - | - | - |
| Dimethylacetamide14 | 2000 | 7 | Toxic hepatitis | Normal | - | - | - |
| Methylene Chloride15 | 2003 | 1 | Toxic hepatitis | Enhanced intrahepatic echo, GB wall thickening | - | Enhanced echo, hepatomegaly, GB wall thickening | - |
| Dimethylformamide16 | 2003 | 1 | Toxic hepatitis | Diffuse hepatocellular disease | - | - | - |
| Trichloroethylene17 | 2005 | 3 | Toxic hepatitis, Stevens-Johnson syndrome | Hepatomegaly | - | - | Hepatocellular necrosis, intracellular cholestasis, ballooning degeneration |
| Chloroform8 | 2010 | 1 | Toxic hepatitis | - | - | Normal | - |
| Chloroform9 | 2014 | 2 | Toxic hepatitis | Normal | - | - | - |
| Mild fatty liver | |||||||
| HCFC-12318 | 2017 | 2 | Toxic hepatitis | - | - | - | Portal and panlobular infiltration of mixed inflammatory cells, multifocal spotty and confluent necrosis, minimal macrovesicular and microvesicular steatosis |
Abbreviations
AST
ALT
CT
DB
GB
γ-GTP
HAV
IgG
INR
MSDS
PT
SWV
TB
TWA
-
Competing interests: The authors declare that they have no competing interests.
-
Author contributions:
NOTES
- 1. Komulainen H. Experimental cancer studies of chlorinated by-products. Toxicology 2004;198(1-3):239–248. 15138047.ArticlePubMed
- 2. Watts P, Long G, Meek ME. Concise international chemical assessment document; 58. Updated 2004]. Accessed July 3, 2022]. https://inchem.org/documents/cicads/cicads/cicad58.htm .
- 3. Brautbar N, Williams J 2nd. Industrial solvents and liver toxicity: risk assessment, risk factors and mechanisms. Int J Hyg Environ Health 2002;205(6):479–491. 12455270.ArticlePubMed
- 4. Bomski H, Sobolewska A, Strakowski A. Toxische schädigung der leber durch chloroform bei chemiebetriebswerkern. Int Arch Gewerbepathol Gewerbehyg 1967;24(2):127–134.ArticlePDF
- 5. Bai C, Canfield PJ, Stacey NH. Individual serum bile acids as early indicators of carbon tetrachloride- and chloroform-induced liver injury. Toxicology 1992;75(3):221–234. 1455431.ArticlePubMed
- 6. Gambini G, Farina G. Hepatic function in workers exposed to inhalation of chloroform vapors. Med Lav 1973;64(11):432–436. 4793806.PubMed
- 7. Phoon WH, Goh KT, Lee LT, Tan KT, Kwok SF. Toxic jaundice from occupational exposure to chloroform. Med J Malaysia 1983;38(1):31–34. 6633331.PubMed
- 8. Lee DG, Lee CH, Jang KH, Chae HJ, Moon JD. A suspicious case of chloroform induced acute toxic hepatitis in laboratory worker. Korean J Occup Environ Med 2012;24(3):304–310.ArticlePDF
- 9. Kang YJ, Ahn J, Hwang YI. Acute liver injury in two workers exposed to chloroform in cleanrooms: a case report. Ann Occup Environ Med 2014;26(1):49. 25411642.ArticlePubMedPMCPDF
- 10. Gemma S, Vittozzi L, Testai E. Metabolism of chloroform in the human liver and identification of the competent P450s. Drug Metab Dispos 2003;31(3):266–274. 12584152.ArticlePubMed
- 11. Malaguarnera G, Cataudella E, Giordano M, Nunnari G, Chisari G, Malaguarnera M. Toxic hepatitis in occupational exposure to solvents. World J Gastroenterol 2012;18(22):2756–2766. 22719183.ArticlePubMedPMC
- 12. Clark JH, Tavener SJ. Alternative solvents: shades of green. Org Process Res Dev 2007;11(1):149–155.Article
- 13. Chae HJ, Lee SK, Lee KJ, Kim JY, Lee SC, Shin DH, et al. Exfoliative dermatitis and toxic hepatitis associated with occupational exposure to trichloroethylene. Korean J Occup Environ Med 2003;15(1):111–117.ArticlePDF
- 14. Choi TS, Woo KH, Kim JS, Park WS, Ham JH, Jung SJ, et al. Toxic hepatitis induced by occupational dimethylacetamide exposure. Korean J Occup Environm Med 2001;13(2):164–170.ArticlePDF
- 15. Ha BG, Kim JS, Yu JY, Woo KH, Ham JO, Yoon SY, et al. A case of toxic hepatitis in a worker exposed to a cleansing agent mainly composed of methylene chloride. Korean J Occup Environ Med 2004;16(2):210–219.ArticlePDF
- 16. Roh JR, Sohn JG, Kim JH, Park SJ. A case of acute toxic hepatitis induced by brief exposure to dimethylformamide. Korean J Occup Environ Med 2005;17(2):144–148.ArticlePDF
- 17. Lee SW, Kim EA, Kim DS, Koh DH, Kang SK, Kim BK, et al. Exposure level of trichloroethylene in Stevens-Johnson syndrome due to occupational exposure: 3 case reports and a review of other cases. Korean J Occup Environ Med 2008;20(2):132–146.ArticlePDF
- 18. Shin MY, Park JS, Park HD, Lee J. HCFC-123-induced toxic hepatitis and death at a Korean fire extinguisher manufacturing facility: a case series. Ann Occup Environ Med 2018;30(1):20. 29610668.ArticlePubMedPMCPDF
REFERENCES
REFERENCES
Figure & Data
REFERENCES
Citations

- Occupational disease monitoring by the Korea Occupational Disease
Surveillance Center: a narrative review
Dong-Wook Lee, Inah Kim, Jungho Hwang, Sunhaeng Choi, Tae-Won Jang, Insung Chung, Hwan-Cheol Kim, Jaebum Park, Jungwon Kim, Kyoung Sook Jeong, Youngki Kim, Eun-Soo Lee, Yangwoo Kim, Inchul Jeong, Hyunjeong Oh, Hyeoncheol Oh, Jea Chul Ha, Jeehee Min, Chul
The Ewha Medical Journal.2025;[Epub] CrossRef - Morbidity of aircraft workers with temporary disability
Olga A. Molchanova, Olga G. Bogdanova, Vladimir A. Pankov, Mikhail Yu. Itygilov
Hygiene and sanitation.2023; 102(8): 836. CrossRef
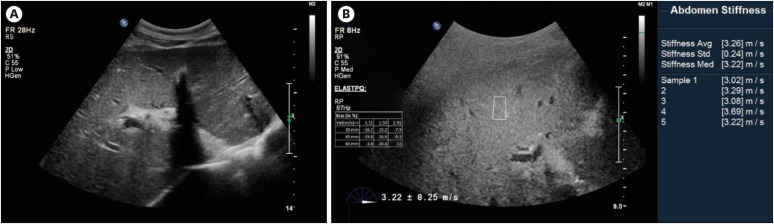
Fig. 1
Fig. 2
Fig. 3
| Date | 22.1.28a | 22.2.4 | 22.2.15 | 22.2.16b | 22.2.17 | 22.2.18 | 22.2.19 | 22.2.20 | 22.2.22 | 22.2.28c | 22.3.15 | Reference range |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total protein (g/dL) | 7.1 | 7.3 | - | - | 5.6 | - | - | 5.1 | 6.1 | 7.1 | - | 6.6–8.3 |
| Albumin (g/dL) | 4.5 | 4.4 | - | 3.9 | 3.3 | 3.1 | 3.1 | 3.0 | 3.7 | 3.8 | - | 3.5–5.2 |
| Total bilirubin (mg/dL) | 9.41 | 5.5 | 18.8 | 21.9 | 24.0 | 24.5 | 24.7 | 23.7 | 22.4 | 11.7 | 5.1 | 0.2–1.2 |
| Direct bilirubin (mg/dL) | 6.77 | 2.7 | 9.9 | 12.9 | 13.4 | 13.9 | 14.0 | 13.6 | 12.1 | 5.4 | 2.2 | 0.0–0.4 |
| AST (IU/L) | 1,140 | 662 | 937 | 1,324 | 1,106 | 756 | 567 | 387 | 240 | 107 | 31 | 0–40 |
| ALT (IU/L) | 2,650 | 482 | 409 | 451 | 408 | 367 | 324 | 269 | 228 | 83 | 13 | 0–40 |
| γ-GTP (IU/L) | 215 | 99 | 88 | - | 79 | 77 | 75 | 70 | 68 | 53 | - | 9–64 |
| PT (seconds) | 13.6 | 13.9 | - | 23.3 | 25.4 | 25.1 | 24.2 | 23.0 | 18.3 | 14.6 | 13.7 | 10.8–14.0 |
| PT (INR) | 1.23 | 1.18 | - | 2.00 | 2.19 | 2.16 | 2.08 | 1.98 | 1.56 | 1.24 | 1.16 | 0.85–1.20 |
| Exposed chemicals | Year | Number of patients | Diagnosis | Ultrasonography | Elastography | CT | Biopsy |
|---|---|---|---|---|---|---|---|
| Trichloroethylene | 1999 | 1 | Toxic hepatitis, exfoliative dermatitis | Enhanced intrahepatic echo | - | - | - |
| Dimethylacetamide | 2000 | 7 | Toxic hepatitis | Normal | - | - | - |
| Methylene Chloride | 2003 | 1 | Toxic hepatitis | Enhanced intrahepatic echo, GB wall thickening | - | Enhanced echo, hepatomegaly, GB wall thickening | - |
| Dimethylformamide | 2003 | 1 | Toxic hepatitis | Diffuse hepatocellular disease | - | - | - |
| Trichloroethylene | 2005 | 3 | Toxic hepatitis, Stevens-Johnson syndrome | Hepatomegaly | - | - | Hepatocellular necrosis, intracellular cholestasis, ballooning degeneration |
| Chloroform | 2010 | 1 | Toxic hepatitis | - | - | Normal | - |
| Chloroform | 2014 | 2 | Toxic hepatitis | Normal | - | - | - |
| Mild fatty liver | |||||||
| HCFC-123 | 2017 | 2 | Toxic hepatitis | - | - | - | Portal and panlobular infiltration of mixed inflammatory cells, multifocal spotty and confluent necrosis, minimal macrovesicular and microvesicular steatosis |
aBefore admission; bOn admission; cDischarge & follow-up.
AST: aspartate aminotransferase; ALT: alanine aminotransferase; γ-GTP: gamma glutamyl transpeptidase; PT: prothrombin time; INR: international normalized ratio.
GB: gallbladder; CT: computed tomography.
 KSOEM
KSOEM

 Cite
Cite

