Articles
- Page Path
- HOME > Ann Occup Environ Med > Volume 30; 2018 > Article
- Research Article Changes of hemodynamic and cerebral oxygenation after exercise in normobaric and hypobaric hypoxia: associations with acute mountain sickness
-
Tobias Kammerer1,2,5
 , Valentina Faihs1, Nikolai Hulde5, Andreas Bayer1, Max Hübner1,2, Florian Brettner1,2, Walter Karlen4, Julia Maria Kröpfl3, Markus Rehm1, Christina Spengler3, Simon Thomas Schäfer1,2
, Valentina Faihs1, Nikolai Hulde5, Andreas Bayer1, Max Hübner1,2, Florian Brettner1,2, Walter Karlen4, Julia Maria Kröpfl3, Markus Rehm1, Christina Spengler3, Simon Thomas Schäfer1,2 -
Annals of Occupational and Environmental Medicine 2018;30:66.
DOI: https://doi.org/10.1186/s40557-018-0276-2
Published online: November 19, 2018
1Department of Anaesthesiology, University Hospital, LMU Munich, Marchioninistr. 15, 81377 Munich, Germany
2Walter Brendel Centre of Experimental Medicine, LMU Munich, Marchioninistr. 15, 81377 Munich, Germany
3Exercise Physiology Lab, Institute of Human Movement Sciences and Sport, ETH Zurich, Winterthurerstr. 190, 8057 Zurich, Switzerland
4Mobile Health Systems Lab, Institute of Robotics and Intelligent Systems, ETH Zurich, Lengghalde 5, 8092 Zurich, Switzerland
5Institute of Anesthesiology, Heart and Diabetes Center NRW, Ruhr University Bochum, Georgstr. 11, 32545 Bad Oeynhausen, Germany
© The Author(s). 2018
Open AccessThis article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Abstract
-
Objective Normobaric (NH) and hypobaric hypoxia (HH) are associated with acute mountain sickness (AMS) and cognitive dysfunction. Only few variables, like heart-rate-variability, are correlated with AMS. However, prediction of AMS remains difficult. We therefore designed an expedition-study with healthy volunteers in NH/HH to investigate additional non-invasive hemodynamic variables associated with AMS.
-
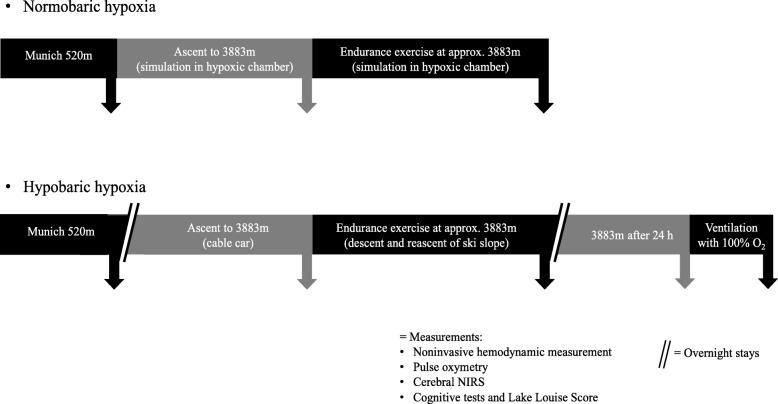
Methods Eleven healthy subjects were examined in NH (FiO2 13.1%; equivalent of 3.883 m a.s.l; duration 4 h) and HH (3.883 m a.s.l.; duration 24 h) before and after an exercise of 120 min. Changes in parameters of electrical cardiometry (cardiac index (CI), left-ventricular ejection time (LVET), stroke volume (SV), index of contractility (ICON)), near-infrared spectroscopy (cerebral oxygenation, rScO2), Lake-Louise-Score (LLS) and cognitive function tests were assessed. One-Way-ANOVA, Wilcoxon matched-pairs test, Spearman’s-correlation-analysis and Student’s t-test were performed.
-
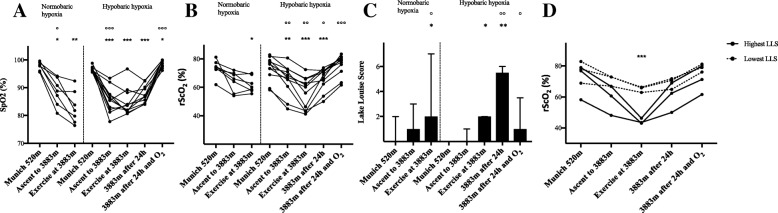
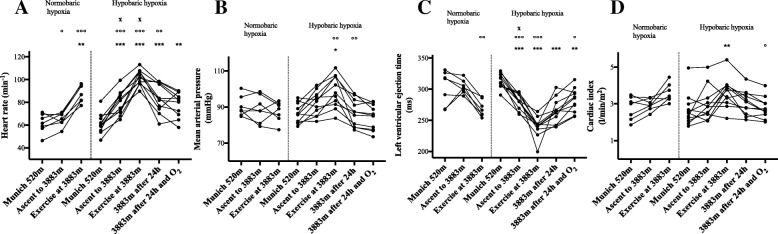
Results HH increased heart rate (HR), mean arterial pressure (MAP) and CI and decreased LVET, SV and ICON, whereas NH increased HR and decreased LVET. In both NH and HH cerebral oxygenation decreased and LLS increased significantly. After 24 h in HH, 6 of 11 subjects (54.6%) developed AMS. LLS remained increased until 24 h in HH, whereas cognitive function remained unaltered. In HH, HR and LLS were inversely correlated (r = − 0.692; p < 0.05). More importantly, the rScO2-decrease after exercise in NH significantly correlated with LLS after 24 h in HH (r = − 0.971; p < 0.01) and rScO2 correlated significantly with HR (r = 0.802; p < 0.01), CI (r = 0.682; p < 0.05) and SV (r = 0.709; p < 0.05) after exercise in HH.
-
Conclusions Both acute NH and HH altered hemodynamic and cerebral oxygenation and induced AMS. Subjects, who adapted their CI had higher rScO2 and lower LLS. Furthermore, rScO2 after exercise under normobaric conditions was associated with AMS at high altitudes.
Introduction
Materials and methods
Results
Discussion
Conclusion
Acknowledgements
- 1. Pramsohler S, Wimmer S, Kopp M, Gatterer H, Faulhaber M, Burtscher M, et al. Normobaric hypoxia overnight impairs cognitive reaction time. BMC Neurosci 2017;18(1):43. 10.1186/s12868-017-0362-3. 28506292.ArticlePubMedPMCPDF
- 2. Asmaro D, Mayall J, Ferguson S. Cognition at altitude: impairment in executive and memory processes under hypoxic conditions. Aviat Space Environ Med 2013;84(11):1159–1165. 10.3357/ASEM.3661.2013. 24279229.ArticlePubMed
- 3. Davranche K, Casini L, Arnal PJ, Rupp T, Perrey S, Verges S. Cognitive functions and cerebral oxygenation changes during acute and prolonged hypoxic exposure. Physiol Behav 2016;164(Pt A):189–197. 10.1016/j.physbeh.2016.06.001. 27262217.ArticlePubMed
- 4. de Aquino Lemos V, Antunes HK, dos Santos RV, Lira FS, Tufik S, de Mello MT. High altitude exposure impairs sleep patterns, mood, and cognitive functions. Psychophysiology 2012;49(9):1298–1306. 10.1111/j.1469-8986.2012.01411.x. 22803634.ArticlePubMed
- 5. Canoui-Poitrine F, Veerabudun K, Larmignat P, Letournel M, Bastuji-Garin S, Richalet JP. Risk prediction score for severe high altitude illness: a cohort study. PLoS One 2014;9(7):e100642. 10.1371/journal.pone.0100642. 25068815.ArticlePubMedPMC
- 6. Richalet JP, Larmignat P, Poitrine E, Letournel M, Canoui-Poitrine F. Physiological risk factors for severe high-altitude illness: a prospective cohort study. Am J Respir Crit Care Med 2012;185(2):192–198. 10.1164/rccm.201108-1396OC. 22071330.ArticlePubMed
- 7. Schneider M, Bernasch D, Weymann J, Holle R, Bartsch P. Acute mountain sickness: influence of susceptibility, preexposure, and ascent rate. Med Sci Sports Exerc 2002;34(12):1886–1891. 10.1097/00005768-200212000-00005. 12471292.ArticlePubMed
- 8. Sutherland A, Freer J, Evans L, Dolci A, Crotti M, Macdonald JH. MEDEX 2015: heart rate variability predicts development of Acute Mountain sickness. High Alt Med Biol 2017;18(3):199–208. 10.1089/ham.2016.0145. 28418725.ArticlePubMed
- 9. Dykiert D, Hall D, van Gemeren N, Benson R, Der G, Starr JM, et al. The effects of high altitude on choice reaction time mean and intra-individual variability: results of the Edinburgh altitude research expedition of 2008. Neuropsychology 2010;24(3):391–401. 10.1037/a0018502. 20438216.ArticlePubMed
- 10. Komiyama T, Sudo M, Higaki Y, Kiyonaga A, Tanaka H, Ando S. Does moderate hypoxia alter working memory and executive function during prolonged exercise? Physiol Behav 2015;139:290–296. 10.1016/j.physbeh.2014.11.057. 25460539.ArticlePubMed
- 11. Virues-Ortega J, Buela-Casal G, Garrido E, Alcazar B. Neuropsychological functioning associated with high-altitude exposure. Neuropsychol Rev 2004;14(4):197–224. 10.1007/s11065-004-8159-4. 15796116.ArticlePubMedPDF
- 12. Roach R, Bartsch P, Hackett P, Oelz O. The Lake Louise acute mountain sickness scoring system. Hypoxia and molecular medicine 1993;272:4.
- 13. Osypka MJ, Bernstein DP. Electrophysiologic principles and theory of stroke volume determination by thoracic electrical bioimpedance. AACN Clin Issues 1999;10(3):385–399. 10.1097/00044067-199908000-00008. 10745708.ArticlePubMed
- 14. Millet GP, Faiss R, Point PV. Hypobaric hypoxia induces different physiological responses from normobaric hypoxia. J Appl Physiol 2012;112(10):1783–1784. 10.1152/japplphysiol.00067.2012. 22267386.PubMed
- 15. Conkin J, Wessel JH 3rd. Critique of the equivalent air altitude model. Aviat Space Environ Med 2008;79(10):975–982. 10.3357/ASEM.2331.2008. 18856188.ArticlePubMed
- 16. Loeppky JA, Roach RC, Maes D, Hinghofer-Szalkay H, Roessler A, Gates L, et al. Role of hypobaria in fluid balance response to hypoxia. High Alt Med Biol 2005;6(1):60–71. 10.1089/ham.2005.6.60. 15772501.ArticlePubMed
- 17. Roach RC, Loeppky JA, Icenogle MV. Acute mountain sickness: increased severity during simulated altitude compared with normobaric hypoxia. J Appl Physiol 1996;81(5):1908–1910. 10.1152/jappl.1996.81.5.1908. 8941508.ArticlePubMed
- 18. Beidleman BA, Muza SR, Fulco CS, Cymerman A, Ditzler D, Stulz D, et al. Intermittent altitude exposures reduce acute mountain sickness at 4300 m. Clin Sci (Lond) 2004;106(3):321–328. 10.1042/CS20030161. 14561214.ArticlePubMedPDF
- 19. Fulco CS, Muza SR, Beidleman BA, Demes R, Staab JE, Jones JE, et al. Effect of repeated normobaric hypoxia exposures during sleep on acute mountain sickness, exercise performance, and sleep during exposure to terrestrial altitude. Am J Physiol Regul Integr Comp Physiol 2011;300(2):R428–R436. 10.1152/ajpregu.00633.2010. 21123763.ArticlePubMed
- 20. Stray-Gundersen J, Chapman RF, Levine BD. “Living high-training low” altitude training improves sea level performance in male and female elite runners. J Appl Physiol 2001;91(3):1113–1120. 10.1152/jappl.2001.91.3.1113. 11509506.ArticlePubMed
- 21. Schommer K, Wiesegart N, Menold E, Haas U, Lahr K, Buhl H, et al. Training in normobaric hypoxia and its effects on acute mountain sickness after rapid ascent to 4559 m. High Alt Med Biol 2010;11(1):19–25. 10.1089/ham.2009.1019. 20367484.ArticlePubMed
- 22. Levine BD. Going high with heart disease: the effect of high altitude exposure in older individuals and patients with coronary artery disease. High Alt Med Biol 2015;16(2):89–96. 10.1089/ham.2015.0043. 26060882.ArticlePubMed
- 23. Issa AN, Herman NM, Wentz RJ, Taylor BJ, Summerfield DC, Johnson BD. Association of Cognitive Performance with time at altitude, sleep quality. and Acute Mountain Sickness Symptoms Wilderness Environ Med 2016;27(3):371–378. 10.1016/j.wem.2016.04.008. 27460198.PubMed
REFERENCES
Notes
Figure & Data
REFERENCES
Citations

- Acute high-altitude illness: risk factors, susceptibility prediction, and personalized prevention and treatment
Nan Jia, Chen Chen, Qian Chen, Junling Liu, Zherui Shen, Yuhan Liu, Caixia Pei, Yilan Wang, Demei Huang, Fei Wang, Yacong He, Zhenxing Wang
Frontiers in Medicine.2026;[Epub] CrossRef - Effects of different exposures to normobaric hypoxia on cognitive performance in healthy young adults.
María Ramírez-delaCruz, David Ortiz-Sánchez, Alfredo Bravo-Sánchez, Javier Portillo, Paula Esteban-García, Javier Abián-Vicén
Physiology & Behavior.2025; 288: 114747. CrossRef - High Altitude Dynamics in Cerebral Oxygenation of Mountain Rescue Personnel: A Prospective Alpine Proof-of-Concept Field Study
Sebastian Schnaubelt, Alexander Egger, Verena Fuhrmann, Katharina Tscherny, Maximilian Niederer, Thomas Uray, Wolfgang Schreiber, Harald Herkner, Dominik Roth
Prehospital and Disaster Medicine.2025; 40(1): 33. CrossRef - Production of IL-1β and IL-10 by Blood Cells of Rats before and One Month after Sublethal Hypoxic Exposure in a Decompression Chamber
D. Sh. Dzhalilova, A. M. Kosyreva, M. V. Silina, M. A. Mayak, I. S. Tsvetkov, O. V. Makarova
Bulletin of Experimental Biology and Medicine.2025; 178(5): 670. CrossRef - Closing the loop: autonomous intelligent control for hypoxia pre-acclimatization and high-altitude health management
Dawei Shi, Jing Chen, Meitong Li, Lingling Zhu, Xunming Ji
National Science Review.2025;[Epub] CrossRef - Physiological and molecular mechanisms of tolerance to hypoxia and oxygen deficiency resistance markers
Maria Silina, Dzhuliia Dzhalilova, Nikolai Fokichev, Olga Makarova
Frontiers in Molecular Biosciences.2025;[Epub] CrossRef - Morphofunctional Features of the Immune System Response to Sublethal Hypoxic Load in Hypoxia-Tolerant and Hypoxia-Susceptible Animals
Maria Kirillova, Dzhuliia Dzhalilova, Mariia Zubareva, Nikolai Fokichev, Olga Makarova
Biomedicines.2025; 13(12): 3022. CrossRef - Noninvasive identification and therapeutic implications of supernormal left ventricular contractile phenotype
Yi Wang, Lixue Yin
Exploration of Cardiology.2024; 2(3): 97. CrossRef - Can acute high-altitude sickness be predicted in advance?
Yan Guo, Xiao Liu, Qiang Zhang, Zhongshan Shi, Menglan Zhang, Jie Chen
Reviews on Environmental Health.2024; 39(1): 27. CrossRef - Association between high cardiac output at altitude and acute mountain sickness: preliminary study on Mt. Fuji
Takeshi Ebihara, Kentaro Shimizu, Yumi Mitsuyama, Hiroshi Ogura, Jun Oda
Journal of Physiological Anthropology.2023;[Epub] CrossRef - Sensitivity of cognitive function tests to acute hypoxia in healthy subjects: a systematic literature review
Titiaan E. Post, Laurens G. Heijn, Jens Jordan, Joop M. A. van Gerven
Frontiers in Physiology.2023;[Epub] CrossRef - Combining hypoxia with thermal stimuli in humans: physiological responses and potential sex differences
Seaver O. Wait, Nisha Charkoudian, Jared W. Skinner, Caroline J. Smith
American Journal of Physiology-Regulatory, Integrative and Comparative Physiology.2023; 324(6): R677. CrossRef - Contribution of Hypoxic Exercise Testing to Predict High-Altitude Pathology: A Systematic Review
Thomas Georges, Pierre Menu, Camille Le Blanc, Sophie Ferreol, Marc Dauty, Alban Fouasson-Chailloux
Life.2022; 12(3): 377. CrossRef - The effects of normobaric and hypobaric hypoxia on cognitive performance and physiological responses: A crossover study
Erich Hohenauer, Livia Freitag, Joseph T. Costello, Thomas B. Williams, Thomas Küng, Wolfgang Taube, Miriam Herten, Ron Clijsen, Shigehiko Ogoh
PLOS ONE.2022; 17(11): e0277364. CrossRef - Changes in prefrontal cerebral oxygenation and microvascular blood volume in hypoxia and possible association with acute mountain sickness
Giorgio Manferdelli, Mauro Marzorati, Chris Easton, Simone Porcelli
Experimental Physiology.2021; 106(1): 76. CrossRef - Differences in Tolerance to Hypoxia: Physiological, Biochemical, and Molecular-Biological Characteristics
Dzhuliia Dzhalilova, Olga Makarova
Biomedicines.2020; 8(10): 428. CrossRef - The interactive effects of acute exercise and hypoxia on cognitive performance: A narrative review
Soichi Ando, Takaaki Komiyama, Mizuki Sudo, Yasuki Higaki, Koji Ishida, Joseph T. Costello, Keisho Katayama
Scandinavian Journal of Medicine & Science in Sports.2020; 30(3): 384. CrossRef - Acute Exercise in Hypobaric Hypoxia Attenuates Endothelial Shedding in Subjects Unacclimatized to High Altitudes
Julia M. Kröpfl, Tobias Kammerer, Valentina Faihs, Hans-Jürgen Gruber, Jan Stutz, Markus Rehm, Ingeborg Stelzer, Simon T. Schäfer, Christina M. Spengler
Frontiers in Physiology.2020;[Epub] CrossRef - Hypoxic-Inflammatory Responses under Acute Hypoxia: In Vitro Experiments and Prospective Observational Expedition Trial
Tobias Kammerer, Valentina Faihs, Nikolai Hulde, Manfred Stangl, Florian Brettner, Markus Rehm, Mareike Horstmann, Julia Kröpfl, Christina Spengler, Simone Kreth, Simon Schäfer
International Journal of Molecular Sciences.2020; 21(3): 1034. CrossRef - The effects of environmental hypoxia on substrate utilisation during exercise: a meta-analysis
Alex Griffiths, Oliver M. Shannon, Jamie Matu, Roderick King, Kevin Deighton, John P. O’Hara
Journal of the International Society of Sports Nutrition.2019;[Epub] CrossRef
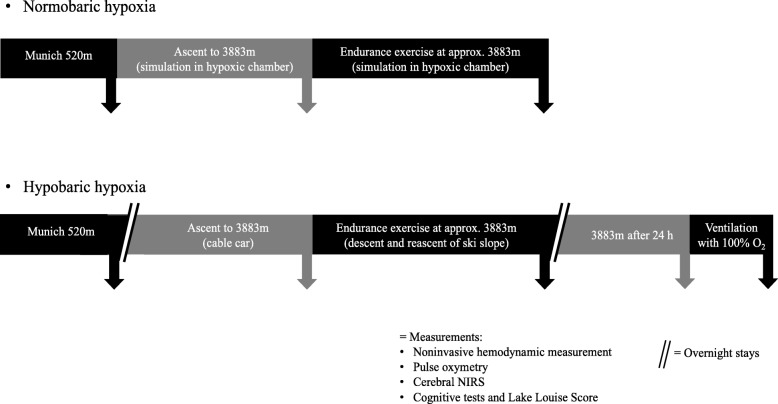
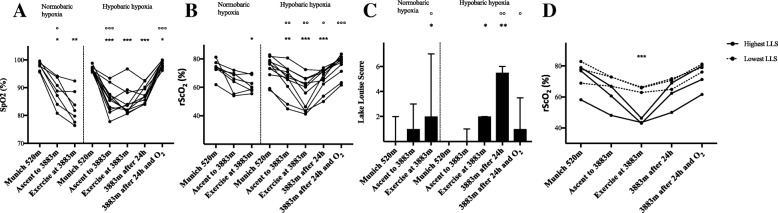
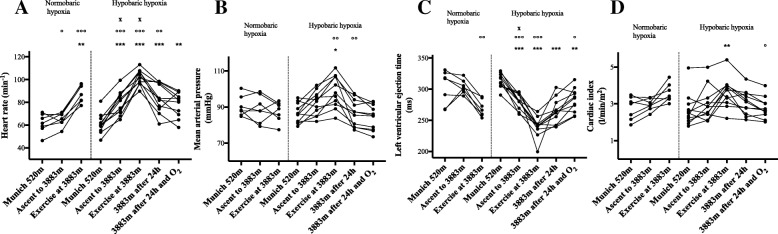
Fig. 1
Fig. 2
Fig. 3
| Normobaric hypoxia (hypoxic chamber) | Hypobaric hypoxia (Little Matterhorn summit) | |||||||
|---|---|---|---|---|---|---|---|---|
| Munich 520 m | Ascent to simulated 3883 m | Exercise at simulated 3883 m | Munich 520 m | Ascent to 3883 m | Exercise at 3883 m | 3883 m after 24 h | 3883 m after 24 h and O2 | |
| Cardiac index (l/min/m2) | 2.8 ± 0.6 | 3.0 ± 0.3 | 3.6 ± 0.50 | 2.7 ± 0.9 | 3.0 ± 0.9 | 3.6 ± 0.8** | 3.1 ± 0.7 | 2.8 ± 0.6 |
| Cardiac output (l/min) | 5.2 ± 0.8 | 5.6 ± 0.5 | 6.8 ± 0.8 | 5.0 ± 1.5 | 5.8 ± 1.6 | 6.9 ± 1.5 | 5.8 ± 1.4 | 5.3 ± 1.2 |
| Stroke volume (ml) | 84.3 ± 7.8 | 85.3 ± 7.6 | 76.5 ± 8.4 | 81.4 ± 14.0 | 74.1 ± 14.0 | 68.0 ± 10.7* | 67.6 ± 13.5* | 69.6 ± 13.6 |
| Left ventricular ejection time (ms) | 303 ± 27 | 304 ± 11 | 267 ± 13 | 313 ± 12 | 281 ± 12*** | 240 ± 16*** | 264 ± 20*** | 282 ± 19** |
| Index of contractility | 68.8 ± 20.1 | 68.2 ± 8.0 | 53.1 ± 13.8 | 64.8 ± 22.6 | 50.0 ± 17.5 | 45.2 ± 17.1** | 44.0 ± 15.2** | 45.9 ± 13.6* |
Values are presented as mean ± SD. Statistical analysis with one-way repeated-measures ANOVA with Greenhouse-Geisser correction, followed by multiple comparisons with Bonferroni correction for multiple comparisons, *
 KSOEM
KSOEM

 Cite
Cite

