Articles
- Page Path
- HOME > Ann Occup Environ Med > Volume 30; 2018 > Article
- Review A brief review of relationship between occupational benzene exposure and hematopoietic cancer
- Jin-Ha Yoon1,2, Woo Seok Kwak2, Yeon-Soon Ahn3
-
Annals of Occupational and Environmental Medicine 2018;30:33.
DOI: https://doi.org/10.1186/s40557-018-0245-9
Published online: May 10, 2018
1Department of Preventive Medicine, Yonsei University College of Medicine, Seoul, Korea
2The Institute for Occupational Health, Yonsei University College of Medicine, Seoul, Korea
3Department of Preventive Medicine, Institute of Occupational and Environmental Medicine, Yonsei University Wonju College of Medicine, 162, Ilsan-dong, Wonju, South Korea, Wonju, 26426 Korea
© The Author(s). 2018
Open AccessThis article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Abstract
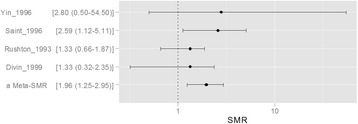
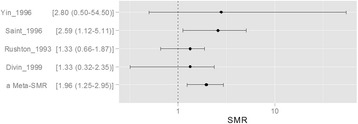
- We reviewed articles to clarify the current evidence status for 1) types of cancer which related to benzene exposure, and 2) certain benzene exposure level which might cause the hematopoietic cancers. Hematopoietic function of the bone marrow is involved in the production of all blood cells types. The benzene metabolites including benzoquinone and mucoaldehyde affect hematopoietic stem cells as well as differentiation steps of progenitor cells for each blood cell. Hence, we concluded that benzene was associated with all lymphohematic carcinogenesis. First, it is supported by biological plausibility. Second, it is supported by meta-analysis although sing study did not show relationship due to lack of sample size or statistical power. More recent studies show lesser exposed level related to risk of cancer, compare to past studies did. Actually, early studies show the risk of malignancies in workers who exposed more than 200 ppm-years. However, only 0.5 to 1 ppm-year benzene exposed show significant linking to risk of malignancies in recent study. As reviewed research articles, we concluded that the relatively lower exposure level, such as 0.5–1 ppm-year, will be considering at risk of hematopoietic cancer. However, more research needs to be done on dose-response analysis.
Background
Main text
Conclusions
Abbreviations
ALL
AML
ANLL
CI
IARC
NIOSH
OSHA
RR
SMR
- 1. McMichael AJ. Carcinogenicity of benzene, toluene and xylene: epidemiological and experimental evidence. IARC Sci Publ 1988;85:3–18.
- 2. http://monographs.iarc.fr/ENG/Monographs/vol100F/index.php.
- 3. Kipen HM, Cody RP, Crump KS, Allen BC, Goldstein BD. Hematologic effects of benzene: a thirty-five year longitudinal study of rubber workers. Toxicol Ind Health 1988;4(4):411–430. 10.1177/074823378800400401. 3188041.ArticlePubMedPDF
- 4. Crump KS. Risk of benzene-induced leukemia: a sensitivity analysis of the pliofilm cohort with additional follow-up and new exposure estimates. J Toxicol Environ Health 1994;42(2):219–242. 10.1080/15287399409531875. 8207757.ArticlePubMed
- 5. Glass DC, Gray CN, Jolley DJ, Gibbons C, Sim MR, Fritschi L, Adams GG, Bisby JA, Manuell R. Leukemia risk associated with low-level benzene exposure. Epidemiology 2003;14(5):569–577. 10.1097/01.ede.0000082001.05563.e0. 14501272.ArticlePubMed
- 6. Glass DC, Gray CN, Jolley DJ, Gibbons C, Sim MR. Health watch exposure estimates: do they underestimate benzene exposure? Chem Biol Interact 2005;153-154:23–32. 10.1016/j.cbi.2005.03.006. 15935797.ArticlePubMed
- 7. https://cfpub.epa.gov/ncea/iris/iris_documents/documents/toxreviews/0276tr.pdf.
- 8. Ross D. The role of metabolism and specific metabolites in benzene-induced toxicity: evidence and issues. J Toxicol Environ Health A 2000;61(5–6):357–372. 10.1080/00984100050166361. 11086940.ArticlePubMed
- 9.
- 10. Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, Lister TA, Bloomfield CD. The World Health Organization classification of hematological malignancies report of the clinical advisory committee meeting, Airlie house, Virginia, November 1997. Mod Pathol 2000;13(2):193–207. 10.1038/modpathol.3880035. 10697278.ArticlePubMedPDF
- 11. Ohishi K, Katayama N, Shiku H, Varnum-Finney B, Bernstein ID. Notch signalling in hematopoiesis. Semin Cell Dev Biol 2003;14(2):143–150. 10.1016/S1084-9521(02)00183-0. 12651098.ArticlePubMed
- 12. Gassmann W, Loffler H, Thiel E, Ludwig WD, Schwartz S, Haferlach T, Maurer J, Rieder H, Fonatsch C, Gokbuget N, Hoelzer D. Morphological and cytochemical findings in 150 cases of T-lineage acute lymphoblastic leukaemia in adults. German multicentre ALL study group (GMALL). Br J Haematol 1997;97(2):372–382. 10.1046/j.1365-2141.1997.d01-2171.x. 9163604.ArticlePubMedPDF
- 13. McHale CM, Zhang L, Smith MT. Current understanding of the mechanism of benzene-induced leukemia in humans: implications for risk assessment. Carcinogenesis 2012;33(2):240–252. 10.1093/carcin/bgr297. 22166497.ArticlePubMedPMC
- 14. Wang L, He X, Bi Y, Ma Q. Stem cell and benzene-induced malignancy and hematotoxicity. Chem Res Toxicol 2012;25(7):1303–1315. 10.1021/tx3001169. 22540379.ArticlePubMed
- 15. Goldstein BD. Benzene as a cause of lymphoproliferative disorders. Chem Biol Interact 2010;184(1–2):147–150. 10.1016/j.cbi.2009.12.021. 20035727.ArticlePubMed
- 16. http://seer.cancer.gov/csr/1975_2010/results_merged/sect_13_leukemia.pdf.
- 17. http://kosis.kr/statisticsList/statisticsList_01List.jsp?vwcd=MT_ZTITLE&parentId=D.
- 18. Infante PF. Benzene exposure and multiple myeloma: a detailed meta-analysis of benzene cohort studies. Ann N Y Acad Sci 2006;1076:90–109. 10.1196/annals.1371.081. 17119195.PubMed
- 19. Wong O. Risk of acute myeloid leukaemia and multiple myeloma in workers exposed to benzene. Occup Environ Med 1995;52(6):380–384. 10.1136/oem.52.6.380. 7627314.ArticlePubMedPMC
- 20. Hayes RB, Yin SN, Dosemeci M, Li GL, Wacholder S, Travis LB, Li CY, Rothman N, Hoover RN, Linet MS. Benzene and the dose-related incidence of hematologic neoplasms in China. Chinese academy of preventive medicine--National Cancer Institute benzene study group. J Natl Cancer Inst 1997;89(14):1065–1071. 10.1093/jnci/89.14.1065. 9230889.PubMed
- 21. Schnatter AR, Glass DC, Tang G, Irons RD, Rushton L. Myelodysplastic syndrome and benzene exposure among petroleum workers: an international pooled analysis. J Natl Cancer Inst 2012;104(22):1724–1737. 10.1093/jnci/djs411. 23111193.ArticlePubMedPMC
- 22.
- 23.
REFERENCES
Notes
Figure & Data
REFERENCES
Citations

- Transcriptomics and proteomics reveals the potential mechanisms of hydroquinone-inhibited erythroid differentiation in K562 cells
Chunhong Yu, Jiaxi Chen, Zetao Zhao, Xinyue Tan, Xinyu Liu, Zongchun Yi
Toxicology Research.2025;[Epub] CrossRef - Environmental and occupational risk factors associated with multiple myeloma: a multicenter, hospital-based, matched case-control study
Mohammad Alnees, Nizar Abu Hamdeh, Ibraheem AbuAlrub, Anwar Zahran, Sari Zraiq, Basem Bali, Fadi Hadya, Osama Ewidat, Duha Najajra, Abdalaziz Darwish, Ruzan Jamaleddin, Mohammed M. H. Qabaha, Moaath Sawalha, Abed Alawna, Saad Allaham, Loay Shaheen, Ezz Al
BMC Public Health.2025;[Epub] CrossRef - Review on novel toxicological effects and personalized health hazard in workers exposed to low doses of benzene
Tongshuai Wang, Yiyi Cao, Zhaolin Xia, David C. Christiani, William W. Au
Archives of Toxicology.2024; 98(2): 365. CrossRef - UBE2L3 promotes benzene-induced hematotoxicity via autophagy-dependent ferroptosis
Boshen Wang, Fei Li, Juan Hu, Fengmei Sun, Lei Han, Juan Zhang, Baoli Zhu
Ecotoxicology and Environmental Safety.2024; 283: 116773. CrossRef - Integrated assessment of inhalation health risk and economic benefit of improving ambient targeted VOCs in Petrochemical industrial area
Wissawa Malakan, Sarawut Thepanondh, Jutarat Keawboonchu, Vanitchaya Kultan, Akira Kondo, Hikari Shimadera
Air Quality, Atmosphere & Health.2024; 17(9): 1885. CrossRef - Genetic prediction of causal association between serum bilirubin and hematologic malignancies: a two-sample Mendelian randomized and bioinformatics study
Lihua Lu, Luting Luo, Xiang Li, Wanying Liu, Boheng Wu, Qing Cai, Jiazheng Li, Yan Huang, Yanxin Chen, Yongzhi Zheng, Jianda Hu
Frontiers in Oncology.2024;[Epub] CrossRef - Investigating the Variation of Benzene and 1,3-Butadiene in the UK during 2000–2020
Rayne Holland, M. Anwar H. Khan, James C. Matthews, Sophia Bonifacio, Rhian Walters, Priya Koria, Joanna Clowes, Karla Rodgers, Temi Jones, Leeya Patel, Rhianna Cross, Freya Sandberg, Dudley E. Shallcross
International Journal of Environmental Research and Public Health.2022; 19(19): 11904. CrossRef - Photocatalytic destruction of volatile aromatic compounds by platinized titanium dioxide in relation to the relative effect of the number of methyl groups on the benzene ring
Jinjian Zhang, Kumar Vikrant, Ki-Hyun Kim, Fan Dong
Science of The Total Environment.2022; 822: 153605. CrossRef - Surface-Modified Wrinkled Mesoporous Nanosilica as an Effective Adsorbent for Benzene, Toluene, Ethylbenzene, and Xylene in Indoor Air
In-Keun Shim, Jeonghoon Kim, Jin Kyu Lee, Jae-Min Oh, Jin Kuen Park
ACS Applied Nano Materials.2022; 5(12): 18138. CrossRef - LincRNA-p21 promotes p21-mediated cell cycle arrest in benzene-induced hematotoxicity by sponging miRNA-17-5p
Boshen Wang, Shouxiang Xu, Tong Wang, Kai Xu, Lihong Yin, Xiaoqin Li, Rongli Sun, Yuepu Pu, Juan Zhang
Environmental Pollution.2022; 296: 118706. CrossRef - Deranged hembiosynthetic pathway in gasoline dispensers in Nigeria: Implications for risk of myeloproliferative disorders and chemoprevention
John Ibhagbemien Anetor, Temidayo Olamide Adigun, Elizabeth Bosede Bolajoko, Gloria Oiyahumen Anetor, Bose Etaniamhe Orimadegun, Moses Olayemi Akiibinu, Godwin Osaretin Igharo, Ayobola Abolape Iyanda, Oluwakemi O. Ademola-Aremu, Chukwuemelie Zedech Uche
American Journal of Biopharmacy and Pharmaceutical Sciences.2022; 2: 2. CrossRef - Evi1 involved in benzene-induced haematotoxicity via modulation of PI3K/mTOR pathway and negative regulation Serpinb2
Rongli Sun, Linling Yu, Kai Xu, Yunqiu Pu, Jiawei Huang, Manman Liu, Juan Zhang, Lihong Yin, Yuepu Pu
Chemico-Biological Interactions.2022; 354: 109836. CrossRef - The shape of low-concentration dose–response functions for benzene: implications for human health risk assessment
Louis A. Cox, Hans B. Ketelslegers, R. Jeffrey Lewis
Critical Reviews in Toxicology.2021; 51(2): 95. CrossRef - Factors Affecting Adverse Health Effects of Gasoline Station Workers
Umakorn Tongsantia, Sunisa Chaiklieng, Pornnapa Suggaravetsiri, Sari Andajani, Herman Autrup
International Journal of Environmental Research and Public Health.2021; 18(19): 10014. CrossRef -
Ayurvedic formulations containing benzoic and ascorbic acids as additives: benzene formation during storage and impact of additives on quality parameters
Priyanka Sharma, Mukesh Maithani, Vikas Gupta, Parveen Bansal
Journal of Complementary and Integrative Medicine.2021; 18(1): 59. CrossRef - Evidence of inter-species swing adsorption between aromatic hydrocarbons
Kumar Vikrant, Ki-Hyun Kim, Jan E. Szulejko, Danil Boukhvalov, Jin Shang, Jörg Rinklebe
Environmental Research.2020; 181: 108814. CrossRef - Health risks associated with benzene formation in health supplements – An appraisal
Priyanka Sharma, Mukesh Maithani, Vikas Gupta, Mayank Yadav, Parveen Bansal
Interdisciplinary Toxicology.2020; 13(2): 49. CrossRef - Deciphering the Impact of Early-Life Exposures to Highly Variable Environmental Factors on Foetal and Child Health: Design of SEPAGES Couple-Child Cohort
Sarah Lyon-Caen, Valérie Siroux, Johanna Lepeule, Philippe Lorimier, Pierre Hainaut, Pascal Mossuz, Joane Quentin, Karine Supernant, David Meary, Laurence Chaperot, Sam Bayat, Flemming Cassee, Sarah Valentino, Anne Couturier-Tarrade, Delphine Rousseau-Ral
International Journal of Environmental Research and Public Health.2019; 16(20): 3888. CrossRef - Cell-specific regulation of Nrf2 during ROS-Dependent cell death caused by 2,3,5-tris(glutathion-S-yl)hydroquinone (TGHQ)
Fengjiao Zhang, Frances M. Munoz, Lanlan Sun, Shuya Zhang, Serrine S. Lau, Terrence J. Monks
Chemico-Biological Interactions.2019; 302: 1. CrossRef
 KSOEM
KSOEM

 Cite
Cite

