Abstract
-
Background
Arsenic is a carcinogenic heavy metal that has a species-dependent health effects and abandoned metal mines are a source of significant arsenic exposure. Therefore, the aims of this study were to analyze urinary arsenic species and their concentration in residents living near abandoned metal mines and to monitor the environmental health effects of abandoned metal mines in Korea.
-
Methods
This study was performed in 2014 to assess urinary arsenic excretion patterns of residents living near abandoned metal mines in South Korea. Demographic data such as gender, age, mine working history, period of residency, dietary patterns, smoking and alcohol use, and type of potable water consumed were obtaining using a questionnaire. Informed consent was also obtained from all study subjects (n = 119). Urinary arsenic species were quantified using high performance liquid chromatography (HPLC) and inductively coupled plasma mass spectrometry (ICP/MS).
-
Results
The geometric mean of urinary arsenic (sum of dimethylarsinic acid, monomethylarsonic acid, As3+, and As5+) concentration was determined to be 131.98 μg/L (geometric mean; 95% CI, 116.72–149.23) while urinary inorganic arsenic (As3+ and As5+) concentration was 0.81 μg/L (95% CI, 0.53–1.23). 66.3% (n = 79) and 21.8% (n = 26) of these samples exceeded ATSDR reference values for urinary arsenic (>100 μg/L) and inorganic arsenic (>10 μg/L), respectively. Mean urinary arsenic concentrations (geometric mean, GM) were higher in women then in men, and increased with age. Of the five regions evaluated, while four regions had inorganic arsenic concentrations less than 0.40 μg/L, one region showed a significantly higher concentration (GM 15.48 μg/L; 95% CI, 7.51–31.91) which investigates further studies to identify etiological factors.
-
Conclusion
We propose that the observed elevation in urinary arsenic concentration in residents living near abandoned metal mines may be due to environmental contamination from the abandoned metal mine.
-
Trial registration
Not Applicable (We do not have health care intervention on human participants).
-
Keywords:
Keywords
Abandoned metal mine; Arsenic; Arsenic species
Background
Arsenic is a naturally occurring element that is widely found in ground water and agricultural products and is one of the most abundant elements in the earth’s crust. Chronic arsenic exposure in humans is associated with diseases such as skin, lung, and hepatic cancer [
1–
3] and the primary sources of human exposure include ingestion of inorganic arsenic from contaminated water [
4], industrial waste, pesticides, and inadequate mine waste disposal [
5–
7]. As most arsenic metabolites are soluble in water, they contaminate river and underground water, and arsenic contamination in ground water is categorized as a serious public health hazard. According to the United States Agency for Toxic Substances and Disease Registry (ATSDR) [
8], the oral route is considered to be a predominant means of arsenic exposure in the general population. Several studies have also reported that exposure to or consumption of, even low levels, arsenic can lead to carcinogenesis [
9–
11]. In the last few decades, many studies have measured urinary arsenic concentration (organic and inorganic arsenic) and many older studies have used urinary arsenic as a biomarker of recent arsenic exposure. This latter approach, however, became obsolete as certain foods also contain organic arsenic which is similarly excreted in urine. As each arsenic species has different physiological and bioactive properties, separation of urinary arsenic metabolites is considered sufficient to both prevent potential overestimation of arsenic concentration and assess health risk [
12,
13].
Metal mining was economically important in during the 19
th century, however, many mines were abandoned because of the change in industrial and economic conditions during the late 1970s [
14]. Such abandoned metal mines have been identified as an important source of environmental heavy metal contamination and elevated levels of these toxic elements are often present in the soil and ground water of various countries [
15,
16]. In many areas of Korea, there is evidence that the uncontrolled abandoning of these metal mines has had a large and lasting impact [
17] as metals and metalloids dissolved from these mines may have contaminated both surface and ground water through solubilization into the surrounding environment. Further, as preventive measures to avoid environmental pollution after closures were not adequately implemented in some of these mines, debris from them, such as spoil heaps and water, remain potential source of environmental contamination. Therefore, this study was evaluated concentrations of urinary arsenic species in residents living near abandoned metal mines in Korea.
Methods
Study subjects and questionnaire
Initially, we selected villages located within 3 km from the abandoned metal mines and the mine is located upstream of the each villages. urinary arsenic was measured in 974 samples obtained from residents living near abandoned metal mines using hydride generation-graphite furnace atomic absorption spectrometry (GFAAS). Subsequently, arsenic species analyses were carried out in a subset of samples with urinary arsenic concentrations in the 90
th percentile. Therefore this study analyzed urinary arsenic concentrations in 119 adults (45.4% male, 54.5% female) from residing near abandoned metal mines identified by the Ministry of Environment, Korea. The study included 19 villages located in East, West, South and Central Korea and was carried out between May and November, 2014. The abandoned mines are located in the Gangwon/Gyeonggi/Inchon (five villages), Daegu/Gyeongbuk (five villages), Busan/Ulsan/Gyeongnam (three villages), Jeonnam/Jeonbuk (three villages), Chungnam/Chungbuk (3 villages) regions of the Korean Peninsula (Fig.
1). These villages were selected as they are the most densely populated and are located within 3 km from the abandoned metal mines. Furthermore, the National Institute Environmental Research (NIER), Korea has conducted previous studies on heavy metals in farmland soil and drainage at abandoned metal mines area. The study was approved by the Institutional Review Board of the Dong-A University (ref. no. 2-1040709-AB-N-01-201404-BR-04-04). Informed consent was obtained from all participants and personal interviews were conducted to acquire demographic and lifestyle information such as age, drinking water source, current dietary habits, ongoing or previous disease, alcohol consumption, smoking status, type of drinking water being used and period of residency in the study area. Any history of working in mines was also obtained.
Fig. 1The five provinces and locations of abandoned metal mines in this study
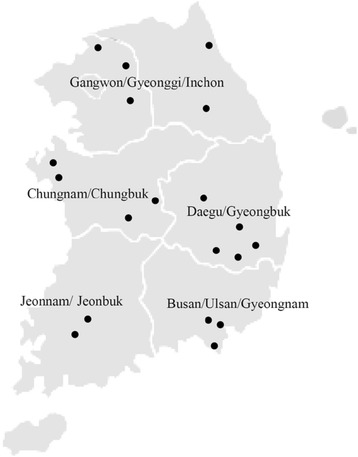
All spot urine samples for organic and inorganic arsenic measurements were collected in disposable urine collection cups, placed in 15 ml polyethylene tubes, maintained at 4 °C, and transported to a laboratory where they were stored at – 70 °C till further analysis.
Urinary arsenic speciation
Urinary arsenic species were analyzed at the Environmental Health Center, Dong-A university. Arsenic species were separated using an inductively coupled plasma mass spectrometry (ICP-MS) instrument (Agilent Technologies, Santa Clara, CA, USA) equipped with a high performance liquid chromatography (HPLC) system (Agilent 1260 Infinity, Agilent Technologies, Santa Clara, CA, USA). This methodology analyses As3+, As5+, monomethylarsonic acid (MMA), and dimethylarsinic acid (DMA) concentrations and the respective limits of detection are 0.17, 0.13, 0.19, and 0.12 μg/L. Urinary arsenic concentration was defined sum of the As3+, As5+, MMA, and DMA concentrations in this study. All urine samples were filtered through a 0.22 μm membrane before being placed into chromatographic vials, and the auto-sampler tray. Five-point calibration curves for all arsenic species tested (As3+, As5+, MMA and DMA) showed satisfactory linearity and the four major arsenic species could be separated within 16 min. The proportion of each arsenic species was calculated by dividing the concentration of that species by urinary arsenic. Replicate analyses of the standard reference material (SRM 2669, NIST standard, quality control) showed coefficient of variation to be less than 8%. For external quality assurance, we also completed both the occupational-medical and environmental-medical programs of German External Quality Assessment Scheme (G-EQUAS) of the Friedrich Alexander University, Erlangen.
Statistical analysis
All statistical analyses were performed using the SAS statistical software (Version 9.4, SAS Institute, Cary, NC). Chi-square test for independence was performed for gender, age, period of residence, smoking, alcohol, type of drinking water, and history of working in mines. Arsenic species concentration is presented as unadjusted and adjusted geometric means with 95% confidence interval due to its right skewed distribution. The ANOVA and t-test were used to examine the relationship between demographic characteristics and arsenic species concentration. A p-value < 0.05 was considered statistically significant.
Results
Characteristics of the study population
The demographic characteristics of the study population are given in Table
1. The average age of the participants was 70.76 ± 9.17 years, and 58% of the participants were aged 70 or above. Among the 119 subjects, 14 subjects (11.8%) were smokers, 17 subjects (14.4%) reported previous history of working in mines, and 64 subjects (68.1%) used underground sources of potable water. Mean period of residence was 50.28 ± 23.77 years and was further categorized into three groups as ≤40, 41–60, and ≥61 years.
Table 1General characteristics, including demographic and lifestyle data, of the study subjects
|
Characteristics |
Total |
Province |
p-value |
|
Gangwon/Kyounggi/Inchon |
Daegu/Gyeongbuk |
Busan/Ulsan/Gyeongnam |
Jeonnam/Jeonbuk |
Chungnam/Chungbuk |
|
Total |
119 |
25(21.0) |
25(21.0) |
14(11.8) |
36(30.3) |
19(16.0) |
|
|
Gender |
Male |
54(45.4) |
7(28.0) |
13(52.0) |
4(28.6) |
19(52.8) |
11(57.9) |
0.132 |
|
Female |
65(54.6) |
18(72.0) |
12(48.0) |
10(71.4) |
17(47.2) |
8(42.1) |
|
|
Age(yr) |
mean ± std |
70.76 ± 9.17 |
71.44 ± 9.51 |
71.12 ± 7.10 |
70.57 ± 7.94 |
68.89 ± 11.01 |
73.11 ± 8.31 |
0.578 |
|
41–59 |
13(10.9) |
2(8.0) |
1(4.0) |
2(14.3) |
7(19.4) |
1(5.3) |
0.541 |
|
60–69 |
37(31.1) |
8(32.0) |
7(28.0) |
3(21.4) |
13(36.1) |
6(31.6) |
|
|
≥70 |
69(58.0) |
15(60.0) |
17(68.0) |
9(64.3) |
16(44.4) |
12(63.2) |
|
|
Period fo residence(yr) |
mean ± std |
50.28 ± 23.77 |
40.04 ± 24.30 |
57.68 ± 21.56 |
52.93 ± 20.53 |
51.92 ± 25.41 |
48.42 ± 22.50 |
0.117 |
|
≤40 |
35(29.7) |
12(50.0) |
3(12.0) |
4(28.6) |
11(30.6) |
5(26.3) |
0.178 |
|
41–60 |
37(31.4) |
7(29.2) |
9(36.0) |
3(21.4) |
10(27.8) |
8(42.1) |
|
|
≥61 |
46(39.0) |
5(20.8) |
13(52.0) |
7(50.0) |
15(41.7) |
6(31.6) |
|
|
Smoking |
Current smoker |
14(11.8) |
2(8.0) |
5(20.0) |
2(14.3) |
5(13.9) |
0 (0.0) |
0.379 |
|
Ex-smoker |
22(18.5) |
3(12.0) |
6(24.0) |
2(14.3) |
5(13.9) |
6(31.6) |
|
|
Non-smoker |
83(69.7) |
20(80.0) |
14(56.0) |
10(71.4) |
26(72.2) |
13(68.4) |
|
|
Alcohol |
Drinking |
64(53.8) |
10(40.0) |
18(72.0) |
5(35.7) |
23(63.9) |
8(42.1) |
0.048 |
|
Non-drinking |
55(46.2) |
15(60.0) |
7(28.0) |
9(64.3) |
13(36.1) |
11(57.9) |
|
|
House income(KRW) |
Below 500,000 |
63(52.9) |
19(76.0) |
11(44.0) |
7(50.0) |
18(50.0) |
8(42.1) |
0.035 |
|
500,000–1,000,000 |
20(16.8) |
1(4.0) |
3(12.0) |
3(21.4) |
11(30.6) |
2(10.5) |
|
|
1,000,000–2,000,000 |
36(30.3) |
5(20.0) |
11(44.0) |
4(28.6) |
7(19.4) |
9(47.4) |
|
|
Mine working history |
Yes |
17(14.4) |
0 (0.0) |
10(40.0) |
1(7.1) |
5(13.9) |
1(5.6) |
0.001 |
|
No |
101(85.6) |
25(100.0) |
15(60.0) |
13(92.9) |
31(86.1) |
17(94.4) |
|
|
Dietary water |
Tap water |
30(31.9) |
3(12.5) |
3(50.0) |
5(41.7) |
10(27.8) |
9(56.3) |
0.037 |
|
Underground water |
64(68.1) |
21(87.5) |
3(50.0) |
7(58.3) |
26(72.2) |
7(43.8) |
|
|
Herbicide |
Yes |
73(70.2) |
13(52.0) |
9(90.0) |
10(71.4) |
26(72.2) |
15(78.9) |
0.158 |
|
No |
31(29.8) |
12(48.0) |
1(10.0) |
4(28.6) |
10(27.8) |
4(21.1) |
|
Data on urinary arsenic species concentration in samples obtained from the five administrative provinces, as mean ± SD and GM with 95% CI, are given in Table
2. DMA was the predominant arsenic species (87.4%) in samples from all provinces, while MMA contributed to 6.5% of the urinary arsenic content. Mean (GM) urinary arsenic concentration, of all subjects, given as sum of As
3+, As
5+, MMA and DMA concentrations, was estimated to be 131.98 μg/L (95% CI, 116.72–149.23 μg/L). Samples from the Jeonnam/Jeonbuk province showed significantly higher urinary arsenic concentration (156.06 μg/L, 95% CI 114.28–213.12 μg/L) than those from other provinces. The concentration of inorganic arsenic species (As
3+ and As
5+) was significantly higher in samples from the Jeonnam/Jeonbuk province, however, levels of organic arsenic (DMA and MMA) were similar among samples from all the provinces. DMA levels contributed to 90–95% of urinary arsenic concentration in all samples except those from the Jeonnam/Jeonbuk province where it was 69%. Data on urinary arsenic species in relation to social-demographic variables are presented in Table
3. Even though urinary arsenic concentrations were higher in women than in men, the difference was not statistically significant, however, DMA concentrations were significantly higher in women than in men (
P = 0.04). DMA concentrations was also higher in subjects with a history of working in mines compared to that in those without, but this difference was not statistically significant (
P = 0.709). In case of period of residence, MMA concentration, same as As
3+ concentration, showed significantly higher (
P = 0.025) with increasing period of residence. Physiologically, As
3+ methylated to form MMA which is further methylated to DMA [
18]. Table
4 gives multivariate-adjusted geometric mean values for urinary arsenic concentration for each province, and urinary arsenic concentrations were similar among the province after adjustment. Inorganic arsenic levels exceeded the maximum reference levels in 8% of the samples from the Gangwon/Kyounggi/Inchon province and in 66.7% of the samples from the Jeonnam/Jeonbuk province (Table
5).
Table 2Distribution of urinary arsenic species concentration (μg/L) in the populations living near abandoned metal mines at each province
|
Province |
Number |
Arsenic species |
GM(95% CI), μg/L |
|
Total |
119 |
Organic |
DMA |
116.11(102.03–132.13) |
|
MMA |
2.08(1.41–3.08) |
|
Inorganic |
As3+
|
0.30(0.20–0.47) |
|
As5+
|
0.37(0.24–0.56) |
|
uAs |
131.98(116.72–149.23) |
|
Gangwon/Kyounggi/Inchon |
25 |
Organic |
DMA |
138.69(112.83–170.47) |
|
MMA |
0.73(0.35–1.52) |
|
Inorganic |
As3+
|
<LOD(0.07–0.29) |
|
As5+
|
<LOD(0.07–0.26) |
|
uAs |
144.39(116.80–178.51) |
|
Daegu/Gyeongbuk |
25 |
Organic |
DMA |
134.92(116.51–156.23) |
|
MMA |
1.66(0.94–2.92) |
|
Inorganic |
As3+
|
<LOD |
|
As5+
|
<LOD |
|
uAs |
138.18(119.59–159.65) |
|
Busan/Ulsan/Gyeongnam |
14 |
Organic |
DMA |
117.29(89.51–153.71) |
|
MMA |
0.73(0.25–2.12) |
|
Inorganic |
As3+
|
<LOD |
|
As5+
|
<LOD |
|
uAs |
119.88(91.22–157.53) |
|
Jeonnam/Jeonbuk |
36 |
Organic |
DMA |
108.36(76.45–153.59) |
|
MMA |
22.61(17.47–29.26) |
|
Inorganic |
As3+
|
4.96(2.31–10.61) |
|
As5+
|
7.10(4.26–11.82) |
|
uAs |
156.06(114.28–213.12) |
|
Chungnam/Chungbuk |
19 |
Organic |
DMA |
85.33(62.65–116.22) |
|
MMA |
0.26(0.14–0.50) |
|
Inorganic |
As3+
|
<LOD |
|
As5+
|
<LOD |
|
uAs |
86.25(63.42–117.30) |
Table 3Unadjusted geometric means of the arsenic species concentration in residents living near abandoned metal mines
|
Characteristics |
Number |
Organic As (μg/L) |
Inorganic As (μg/L) |
Urinary Arsenic (μg/L) |
|
DMA |
MMA |
As3+
|
As5+
|
|
Total |
119 |
116.11(102.03–132.13) |
2.08(1.41–3.08) |
0.30(0.20–0.47) |
0.37(0.24–0.56) |
131.98(116.72–149.23) |
|
Gender |
Male |
54 |
100.55(83.55–121.02) |
2.37(1.32–4.25) |
0.36(0.19–0.69) |
0.45(0.24–0.84) |
118.42(100.40–139.68) |
|
Female |
65 |
130.85(109.42-156.47) |
1.87(1.09–3.20) |
0.27(0.15–0.47) |
0.31(0.18–0.55) |
144.42(120.72–172.76) |
|
p-value |
|
0.044 |
0.550 |
0.486 |
0.396 |
0.112 |
|
Age(yr) |
41-59 |
13 |
95.17(61.56–147.12) |
3.23(0.74–14.03) |
0.54(0.11–2.73) |
0.89(0.21–3.78) |
116.28(76.34–177.12) |
|
60-69 |
37 |
102.00(80.18–129.78) |
2.19(1.01–4.75) |
0.46(0.19–1.08) |
0.54(0.24–1.20) |
121.18(98.10–149.67) |
|
≥70 |
69 |
129.21(109.33–152.70) |
1.86(1.14–3.04) |
0.22(0.13–0.37) |
0.25(0.15–0.43) |
141.50(119.71–167.26) |
|
p-value |
|
0.150 |
0.694 |
0.202 |
0.088 |
0.415 |
|
Period of residence |
≤40 |
35 |
104.43(83.68–130.32) |
0.93(0.41–2.11) |
0.26(0.12–0.56) |
0.34(0.16–0.70) |
117.03(94.82–144.43) |
|
41-60 |
37 |
139.65(111.45–174.99) |
2.39(1.16–4.95) |
0.25(0.11–0.56) |
0.46(0.19–1.11) |
156.52(123.50–198.37) |
|
≥61 |
46 |
107.73(85.53–135.70) |
3.40(1.97–5.87) |
0.41(0.20–0.85) |
0.34(0.18–0.64) |
125.50(102.29–153.98) |
|
p-value |
|
0.154 |
0.025 |
0.581 |
0.781 |
0.159 |
|
Smoking |
Current-smoker |
14 |
79.08(44.38–140.91) |
1.68(0.52–5.41) |
0.20(0.06–0.65) |
0.33(0.11–1.04) |
97.16(64.31–146.79) |
|
Ex-smoker |
22 |
118.72(94.31–149.46) |
2.08(0.79–5.47) |
0.33(0.11–0.99) |
0.32(0.11–0.89) |
133.37(103.50–171.87) |
|
Non-smoker |
83 |
123.15(105.98–143.10) |
2.16(1.34–3.48) |
0.32(0.19–0.54) |
0.39(0.23–0.65) |
138.59(119.22–161.12) |
|
p-value |
|
0.096 |
0.923 |
0.787 |
0.928 |
0.192 |
|
Alcohol |
Drinking |
64 |
102.89(85.73-123.48) |
2.37(1.38-4.07) |
0.34(0.19-0.60) |
0.50(0.28-0.91) |
121.14(103.12-142.31) |
|
Non-drinking |
55 |
133.65(111.54-160.14) |
1.79(1.00-3.20) |
0.27(0.14-0.52) |
0.26(0.14-0.45) |
145.82(120.53-176.42) |
|
p-value |
|
0.045 |
0.478 |
0.602 |
0.108 |
0.137 |
|
Income(KRW) |
Below 500,000 |
63 |
116.52(96.59–140.55) |
2.07(1.21–3.53) |
0.28(0.16–0.50) |
0.38(0.21–0.69) |
131.29(109.08–158.02) |
|
500,000-1,000,000 |
20 |
106.50(71.20–159.29) |
4.28(1.52–12.06) |
0.58(0.17–2.00) |
0.81(0.26–2.48) |
131.59(95.09–182.10) |
|
1,000,000-2,000,000 |
36 |
121.07(100.01–146.55) |
1.40(0.68–2.90) |
0.25(0.11–0.54) |
0.23(0.12–0.44) |
133.42(110.09–161.70) |
|
p-value |
|
0.813 |
0.180 |
0.389 |
0.137 |
0.993 |
|
Mine working history |
Yes |
17 |
124.94(92.57–168.63) |
3.29(1.29–8.38) |
0.32(0.09–1.10) |
0.32(0.10–1.02) |
139.92(105.61–185.38) |
|
No |
101 |
116.66(101.27–134.38) |
1.98(1.28–3.06) |
0.31(0.19–0.49) |
0.38(0.24–0.60) |
133.09(116.44–152.11) |
|
p-value |
|
0.709 |
0.370 |
0.928 |
0.792 |
0.773 |
|
Dietary water |
Tap water |
30 |
118.61(96.99–145.05) |
1.72(0.72–4.10) |
0.27(0.11–0.68) |
0.55(0.22–1.37) |
134.40(108.52–166.46) |
|
Underground water |
64 |
112.52(91.42–138.48) |
2.56(1.44–4.56) |
0.56(0.30–1.05) |
0.55(0.30–1.00) |
133.40(110.23–161.45) |
|
p-value |
|
0.713 |
0.438 |
0.203 |
0.996 |
0.962 |
|
Herbicide |
Yes |
73 |
111.02(93.53–131.78) |
2.22(1.30–3.78) |
0.34(0.19–0.61) |
0.52(0.30–-0.93) |
128.19(108.42–151.56) |
|
No |
31 |
119.48(89.07–160.27) |
1.70(0.75–3.81) |
0.46(0.19–1.13) |
0.33(0.15–0.72) |
137.37(105.42–179.00) |
|
p-value |
|
0.651 |
0.583 |
0.561 |
0.359 |
0.655 |
Table 4Adjusted geometric means for each province
|
Adjusted GM(95% CI), μg/L |
p-value |
|
Total |
Gangwon/Kyounggi/Inchon |
Daegu/Gyeongbuk |
Busan/Ulsan/Gyeongnam |
Jeonnam/Jeonbuk |
Chungnam/Chungbuk |
|
oAs |
DMA |
92.04(63.09–134.28) |
108.01(62.46–186.77) |
106.67(54.03–210.59) |
86.67(48.54–154.76) |
98.25(64.67–149.28) |
58.02(33.86–99.43) |
0.161 |
|
MMA |
4.35(1.36–13.93) |
1.22(0.48-3.11)a
|
0.45(0.14–1.44)ac
|
0.84(0.31–2.24)ac
|
32.80(16.08–66.89)b
|
0.24(0.09–0.59)c
|
<0.001 |
|
Subtotal |
106.62(74.49–152.62) |
113.69(68.39–189.02)a
|
112.00(59.57–210.56)a
|
90.88(53.06–155.66)a
|
124.11(84.17–182.98)a
|
61.56(37.34–101.48)a
|
0.040 |
|
iAs |
As3+
|
0.84(0.23–3.04) |
0.14(0.04–0.46)a
|
0.13(0.03–0.56)a
|
0.07(0.02–0.23)a
|
6.18(2.50–15.32)b
|
0.06(0.02–0.20)a
|
<0.001 |
|
As5+
|
0.83(0.24–2.87) |
0.12(0.04–0.34)a
|
0.10(0.03–0.36)a
|
0.10(0.03–0.28)a
|
6.55(3.03–14.17)b
|
0.05(0.02–0.15)a
|
<0.001 |
|
Subtotal |
1.84(0.53–6.43) |
0.30(0.11–0.76)a
|
0.24(0.07–0.77)a
|
0.15(0.06–0.41)a
|
15.48(7.51–31.91)b
|
0.11(0.04–0.27)a
|
<0.001 |
|
uAs |
115.84(80.17–167.38) |
116.59(70.11–193.87)ac
|
113.73(60.47–213.88)ac
|
91.91(53.64–157.46)ac
|
144.03(97.67–212.39)b
|
61.38(37.22–101.21)c
|
0.008 |
Table 5The number of samples whose levels exceeded the reference levels of inorganic arsenic in each province
|
Number |
Number of exceed reference levels (%)
Inorganic arsenic (>10 μg/L)a
|
|
Total |
119 |
26(21.8) |
|
Province |
Gangwon/Kyounggi/Inchon |
25 |
2(8.0) |
|
Daegu/Gyeongbuk |
25 |
0(0.0) |
|
Busan/Ulsan/Gyeongnam |
14 |
0(0.0) |
|
Jeonnam/Jeonbuk |
36 |
24(66.7) |
|
Chungnam/Chungbuk |
19 |
0(0.0) |
Discussion
This study analyzed urinary arsenic species and their concentration in populations living near abandoned metal mines using HPLC-ICP-MS, and to the best of our knowledge, is the first to do so. The study population comprised only adult, and as participation was voluntary, the demographic data and results presented here are not representative of the general population. A spot urine sample was used for analyses because 24 h urine collection is uncomfortable and often results in improper or incomplete collection. Samples obtained from residents living near one abandoned metal mine located in the Jeonnam/Jeonbuk province alone showed significantly higher concentrations of urinary arsenic and inorganic arsenic. Moreover, life style and demographic status of residents in the Jeonnam/Jeonbuk province was not significantly different compare with other provinces. Potable water and food are important sources of human arsenic exposure, and urinary arsenic concentrations have been reported to be higher in populations living near contaminated areas compared to those residing in uncontaminated area [
19–
21]. Based on the health risks associated with arsenic exposure, the United States Environmental Protection Agency (USEPA) has established a reference level of 10 μg/L for dietary water [
22]. According to the Survey of the Heavy Metals on Farmland Soil and Drainage at Abandoned Mine Area by the National Institute of Environmental Research in Korea (NIER, 2008), arsenic contamination of farmland soil and water within a distance of 2 km from an abandoned metal mine in the Jeonnam/Jeonbuk province exceeded both the preliminary standard and countermeasure standard for arsenic concentration. The Korean National Environmental Health Survey [
23] has reported urinary arsenic concentrations of 35.0 μg/L (GM; 95% CI, 33.8–36.2 μg/L) in the general population aged over 20 years: using the hydride generation method. However, we show that mean urinary arsenic concentration (GM) in populations living near abandoned metal mines is significantly higher than that reported in the NIER survey. This discrepancy could be due to food consumption patterns, as food is an important source of organic arsenic (DMA and MMA) and a possible confounding factor during urinary arsenic species analysis. DMA is found in food as it is the end product of the arsenic metabolic pathway, irrespective of the arsenic species entering the living organism. In addition, seaweed and seafood contain arsenosugars that are converted to DMA after consumption and is excreted as such in urine [
24]. Even though we recommended that all participants refrain from seaweed and seafood consumption for at least 3 days prior to sampling, it could not be strictly enforced. We observed that women had significantly higher DMA concentrations than men (
P = 0.044), probably because available literature suggests that women can more efficiently methylate arsenic compared to men [
25–
27]. Our data show that DMA is the predominant arsenic metabolite in urine (69.2–95%) followed by MMA (1.2–14%), and inorganic arsenic (0.3–7.7%). Previous studies have reported similar results where DMA contributed to 84–86% of the urinary arsenic species [
28,
29]. In 2000, a WHO report estimated that the arsenic content of cigarette smoke was 40–120 ng per cigarette [
30]. However, we observed that smoking was not a significant determinant of urinary arsenic species concentration.
Conclusion
We show that residents living near abandoned metal mines are not markedly overexposed to arsenic except in the case of one abandoned metal mine in the Jeonnam/Jeonbuk province. A probable reason for this observation is consumption of arsenic-contaminated ground water from near the abandoned metal mine.
A limitation of our study is that the arsenic exposed group did not have comparable control subjects. Further, only 10% of the 974 samples were subjected to arsenic species analyses. We, therefore, suggest that all future studies implement arsenic species analysis in all samples rather than only in a subset. We, meanwhile, could not survey the consumption of seafood and seaweed at that time. But, we are going to consider assessment the relationship between arsenic concentration and seafood consumption in the next study. As there are no reports on the analysis of urinary arsenic species using HPLC-ICP-MS in populations living near abandoned metal mines in Korea, this study provides valuable data on the prevalence and concentration of urinary arsenic species in arsenic exposed populations, especially abandoned metal mine area.
Acknowledgements
This study was conducted by the Heavy Metal Exposure Environmental Health Center Research Fund at the Dong-A University supported by the Ministry of Environment, Korea.
Authors’ contributions
JY is the first author of this article and had drafted the manuscript. YS is the corresponding author and JW performed statistical analysis of this article. HJ performed urinary arsenic species analysis using HPLC-ICP-MS. BG, BK, JD, JS, MJ, JD, BS, NS, SD, and BJ contributed to the data collection and exposure assessment. All authors revised this manuscript critically. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This research was approved by the Dong-A University Institutional Review Board (No. 2-1040709-AB-N-01-201404-BR-04-04).
REFERENCES
- 1. Chen CJ, Chen CW, Wu MM, Kuo TL. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer 1992;66(5):888–92. 10.1038/bjc.1992.380. 1419632.ArticlePubMedPMCPDF
- 2. Karagas MR, Gossai A, Pierce B, Ahsan H. Drinking water arsenic contamination, skin lesions, and malignancies: a systematic review of the global evidence. Curr Environ Health Rep 2015;2(1):52–68. 10.1007/s40572-014-0040-x. 26231242.ArticlePubMedPMCPDF
- 3. Roy RV, Son YO, Pratheeshkumar P, Wang L, Hitron JA, Divya SP, et al. Epigenetic targets of arsenic: emphasis on epigenetic modifications during carcinogenesis. J Environ Pathol Toxicol Oncol 2015;34(1):63–84. 10.1615/JEnvironPatholToxicolOncol.2014012066. 25746832.ArticlePubMed
- 4. Mazumder DN. Chronic arsenic toxicity and human health. Indian J Med Res 2008;128(4):436–47. 19106439.PubMed
- 5. Soleo L, Lovreglio P, Iavicoli S, Antelmi A, Drago I, Basso A, et al. Significance of urinary arsenic speciation in assessment of seafood ingestion as the main source of organic and inorganic arsenic in a population resident near a coastal area. Chemosphere 2008;73(3):291–9. 10.1016/j.chemosphere.2008.06.030. 18657289.ArticlePubMed
- 6. Watson WA, Litovitz TL, Rodgers GC Jr, Klein-Schwartz W, Youniss J, Rose SR, et al. 2002 annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med 2003;21(5):353–421. 10.1016/S0735-6757(03)00088-3. 14523881.ArticlePubMed
- 7. Cho Y, Seo S, Choi SH, Lee S, Kim K, Kim HJ, et al. Association of arsenic levels in soil and water with urinary arsenic concentration of residents in the vicinity of closed metal mines. Int J Hyg Environ Health 2013;216(3):255–62. 10.1016/j.ijheh.2012.05.003. 22704486.ArticlePubMed
- 8. Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for arsenic. 2007, Atlanta: U.S. Department of Health and Human Services, Public Health Services.
- 9. Guo HR, Chiang HS, Hu H, Lipsitz SR, Monson RR. Arsenic in drinking water and incidence of urinary cancers. Epidemiology 1997;8(5):545–50. 10.1097/00001648-199709000-00012. 9270957.ArticlePubMed
- 10. Ferreccio C, González C, Milosavjlevic V, Marshall G, Sancha AM, Smith AH. Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology 2000;11(6):673–9. 10.1097/00001648-200011000-00010. 11055628.ArticlePubMed
- 11. Chiou HY, Chiou ST, Hsu YH, Chou YL, Tseng CH, Wei ML, et al. Incidence of transitional cell carcinoma and arsenic in drinking water: a follow-up study of 8,102 residents in an arseniasis-endemic area in northeastern Taiwan. Am J Epidemiol 2001;153(5):411–8. 10.1093/aje/153.5.411. 11226969.ArticlePubMed
- 12. Ma M, Le XC. Effect of arsenosugar ingestion on urinary arsenic speciation. Clin Chem 1998;44(3):539–50. 9510859.ArticlePubMedPDF
- 13. Hakala E, Pyy L. Assessment of exposure to inorganic arsenic by determining the arsenic species excreted in urine. Toxicol Lett 1995;77(1–3):249–58. 10.1016/0378-4274(95)03304-1. 7618147.ArticlePubMed
- 14. Hong YS, Lee BK, Park JD, Sakong J, Choi JW, Moon JD, Kim DS, Kim BG. Blood cadmium concentration of residents living near abandoned metal mines in Korea. J Korean Med Sci 2014;29(5):633–9. 10.3346/jkms.2014.29.5.633. 24851017.ArticlePubMedPMCPDF
- 15. Pierre D, Power MR, Rollinson G, Camm GS, Hughes SH, Butcher AR, et al. The spatial distribution and source of arsenic, copper, tin and zinc within the surface sediments of the Fal Estuary, Cornwall, UK. Sedimentology 2003;50(3):579–95. 10.1046/j.1365-3091.2003.00566.x.Article
- 16. Rieuwerts JS, Mighanetara K, Braungardt CB, Rollinson GK, Pirrie D, Azizi F. Geochemistry and mineralogy of arsenic in mine wastes and stream sediments in a historic metal mining area in the UK. Sci Total Environ 2014;472:226–34. 10.1016/j.scitotenv.2013.11.029. 24295744.ArticlePubMed
- 17.
- 18. Morita M, Edmond JS. Determination of arsenic species in environmental and biological samples. Pure Appl Chem 1992;64(4):575–90. 10.1351/pac199264040575.
- 19. Kavanagh P, Farago ME, Thornton I, Goessler W, Kuehnelt D, Schlagenhaufen C, et al. Urinary arsenic species in Devon and Cornwall residents, UK. A pilot study. Analyst 1998;123(1):27–9. 10.1039/a704893i. 9581016.ArticlePubMed
- 20. Ranft U, Miskovic P, Pesch B, Jakubis P, Fabianova E, Keegan T, et al. Association between arsenic exposure from a coal-burning power plant and urinary arsenic concentrations in Prievidza District, Slovakia. Environ Health Perspect 2003;111(7):889–94. 10.1289/ehp.5838. 12782488.ArticlePubMedPMC
- 21. Hinwood AL, Sim MR, Jolley D, de Klerk N, Bastone EB, Gerostamoulos J, et al. Exposure to inorganic arsenic in soil increases urinary inorganic arsenic concentrations of residents living in old mining areas. Environ Geochem Health 2004;26(1):27–36. 10.1023/B:EGAH.0000020897.15564.93. 15214611.ArticlePubMed
- 22. U.S. EPA (United States Environmental Protection Agency). National Primary Drinking Water Regulations; Arsenic and Clarifications to Compliance and New Source Contaminant Monitoring. Federal Register. 2001.
- 23. Korea National Institute of Environmental Research. The Korean National Environmental Health Survey. 2011.
- 24. Francesconi KA, Tanggaar R, McKenzie CJ, Goessler W. Arsenic metabolites in human urine after ingestion of an arsenosugar. Clin Chem 2002;48(1):92–101. 11751543.ArticlePubMedPDF
- 25. Lindberg AL, Ekström EC, Nermell B, Rahman M, Lönnerdal B, Persson LA, et al. Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environ Res 2008;106(1):110–20. 10.1016/j.envres.2007.08.011. 17900557.ArticlePubMed
- 26. Chung CJ, Huang CJ, Pu YS, Su CT, Huang YK, Chen YT, et al. Urinary 8-hydroxydeoxyguanosine and urothelial carcinoma risk in low arsenic exposure area. Toxicol Appl Pharmacol 2008;226(1):14–21. 10.1016/j.taap.2007.08.021. 17950770.ArticlePubMed
- 27. Hsueh YM, Huang YL, Huang CC, Wu WL, Chen HM, Yang MH, et al. Urinary levels of inorganic and organic arsenic metabolites among residents in an arseniasis-hyperendemic area in Taiwan. J Toxicol Environ Health A 1998;54(6):431–44. 10.1080/009841098158728. 9661909.ArticlePubMed
- 28. Hata A, Endo Y, Nakajima Y, Ikebe M, Ogawa M, Fujitani N, Endo G. PLC-ICP-MS speciation analysis of arsenic in urine of Japanese subjects without occupational exposure. J Occup Health 2007;49(3):217–23. 10.1539/joh.49.217. 17575402.PubMed
- 29. Johnson LR, Farmer JG. Urinary arsenic concentrations and speciation in Cornwall residents. Environ Geochem Health 1989;11(2):39–44. 10.1007/BF01782991. 24202288.ArticlePubMedPDF
- 30. WHO. Air quality guidelines for Europe. WHO Regional Publications, European Series no. 91. 2000, Copenhagen: Regional Office for Europe, World Health Organization.

 KSOEM
KSOEM


 Cite
Cite

