Articles
- Page Path
- HOME > Ann Occup Environ Med > Volume 27; 2015 > Article
- Research Article Association of cadmium with diabetes in middle-aged residents of abandoned metal mines: the first health effect surveillance for residents in abandoned metal mines
- Hee-seung Son, Soo-geun Kim, Byung-seong Suh, Dong-uk Park, Dae-seon Kim, Seung-do Yu, Yeong-seoub Hong, Jung-duck Park, Byung-kook Lee, Jai-dong Moon, Joon Sakong
-
Annals of Occupational and Environmental Medicine 2015;27:20.
DOI: https://doi.org/10.1186/s40557-015-0071-2
Published online: August 24, 2015
Department of 1Occupational and Environmental Medicine, Kangbuk Samsung Hospital, Medical Center of Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
Department of Environmental Health, Korea National Open University, Seoul, Republic of Korea
Environmental Health Research Department, Environmental Health Research Division, Incheon, Republic of Korea
Department of Preventive Medicine, School of Medicine, Dong-A University, Busan, Republic of Korea
Department of Preventive Medicine, College of Medicine, Chung-Ang University, Seoul, Republic of Korea
Korean Industrial Health Association, Hyesan Bldg., Seoul, Republic of Korea
Department of Preventive Medicine and Public Health, College of Medicine, Chonnam National University Hwasun Hospital, Hwasun, Republic of Korea
Department of Preventive Medicine and Public Health, College of Medicine, Yeungnam University, Daegu, Republic of Korea
© Son et al. 2015
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Abstract
-
Objective The aim of this study was to determine the association between urinary cadmium (U-cd) concentration and diabetes in middle-aged Korean residents of abandoned mines using the first Health Effect Surveillance for Residents in Abandoned Metal mines (HESRAM).
-
Methods This study was cross-sectional study conducted on 719 residents between 40–70 years in 38 abandoned metal mines in Korea. Data was collected by HESRAM from 2008 to 2011. The correlation coefficient of U-cd and fasting blood glucose, odds ratio in urinary cadmium tertiles and diabetes prevalence was analyzed according to the sex category.
-
Results The correlation coefficient U-cd concentration and fasting blood glucose was 0.182 in male. Logistic regression analysis in male revealed a third tertile odds ratio of U-cd (2 μg/g creatinine < U-cd) while diabetes prevalence was 1.81 (95 % CI 1.05-3.12) with adjusted age, BMI, smoking and alcohol consumption, region, family income. On the other hand, the odds ratio for third tertile of U-cd (3 μg/g creatinine < U-cd) between diabetes prevalence in female was 1.39 (95 % CI 0.52-3.72) in addition to adjusted menopausal status.
-
Conclusions Environmental exposure to cadmium in abandoned mine residents was associated with diabetes in male. Closed monitoring and periodic evaluation of the health effects of chronic environmental exposure on abandoned mines residents will be needed.
Introduction
Methods
Result
Discussion
Conclusion
-
Competing interests
The authors declare that they have no competing interests.
-
Authors’ contributions
HSS: Study concept and design, Drafting of the manuscript. SGK: Analysis of data. Critical revision of the manuscript. BSS: Technical support and analysis of data. DUP, SDY, TSH, JDP, BKL, JDM, JS: Collecting of data. All authors read and approved the final manuscript.
NOTES
- 1. Coelho P, Silva S, Roma-Torres J, Costa C, Henriques A, Teixeira J, et al. Health impact of living near an abandoned mine--case study: Jales mines. Int J Hyg Environ Health 2007;210(3–4):399–402. 10.1016/j.ijheh.2007.01.004. 17321206.ArticlePubMed
- 2. Henson MC, Chedrese PJ. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp Biol Med 2004;229(5):383–92.ArticlePDF
- 3. Hwangbo Y, Weaver VM, Tellez-Plaza M, Guallar E, Lee BK, Navas-Acien A. Blood cadmium and estimated glomerular filtration rate in Korean adults. Environ Health Perspect 2011;119(12):1800–5. 10.1289/ehp.1003054. 21835726.ArticlePubMedPMC
- 4. Liu J, Qu W, Kadiiska MB. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol 2009;238(3):209–14. 10.1016/j.taap.2009.01.029. 19236887.ArticlePubMedPMC
- 5. Rathmann W, Giani G. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27(10):2568–9. 10.2337/diacare.27.10.2568. 15451946.ArticlePubMedPDF
- 6. Cho NH. The epidemiology of diabetes in Korea: from the economics to genetics. Korean Diabetes J 2010;34(1):10–5. 10.4093/kdj.2010.34.1.10. 20532014.ArticlePubMedPMC
- 7. American Diabetes A. Standards of medical care in diabetes--2010. Diabetes Care 2010;33(Suppl 1):S11–61. 10.2337/dc10-S011. 20042772.ArticlePubMedPMCPDF
- 8. El Muayed M, Raja MR, Zhang X, MacRenaris KW, Bhatt S, Chen X, et al. Accumulation of cadmium in insulin-producing beta cells. Islets 2012;4(6):405–16. 10.4161/isl.23101. 23466887.ArticlePubMedPMC
- 9. Gerson RJ, Shaikh ZA. Uptake and binding of cadmium and mercury to metallothionein in rat hepatocyte primary cultures. Biochem. J. 1982;208(2):465–72. 10.1042/bj2080465. 7159412.ArticlePubMedPMCPDF
- 10. Liuzzi JP, Bobo JA, Lichten LA, Samuelson DA, Cousins RJ. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc Natl Acad Sci U S A 2004;101(40):14355–60. 10.1073/pnas.0406216101. 15381762.ArticlePubMedPMC
- 11. Serdar MA, Bakir F, Hasimi A, Celik T, Akin O, Kenar L, et al. Trace and toxic element patterns in nonsmoker patients with noninsulin-dependent diabetes mellitus, impaired glucose tolerance, and fasting glucose. Int J Diabetes Dev Ctries 2009;29(1):35–40. 10.4103/0973-3930.50713. 20062562.ArticlePubMedPMC
- 12. Schwartz GG, Il’yasova D, Ivanova A. Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care 2003;26(2):468–70. 10.2337/diacare.26.2.468. 12547882.ArticlePubMedPDF
- 13. Moon SS. Association of lead, mercury and cadmium with diabetes in the Korean population: the Korea National Health and Nutrition Examination Survey (KNHANES) 2009–2010. Diabetic Med 2013;30(4):e143–8. 10.1111/dme.12103. 23278294.PubMed
- 14. Elinder CG, Lind B, Kjellstrom T, Linnman L, Friberg L. Cadmium in kidney cortex, liver, and pancreas from Swedish autopsies. Estimation of biological half time in kidney cortex, considering calorie intake and smoking habits. Arch Environ Health 1976;31(6):292–302. 10.1080/00039896.1976.10667239. 999342.PubMed
- 15. Park DU, Kim DS, Yu SD, Lee KM, Ryu SH, Kim SG, et al. Blood levels of cadmium and lead in residents near abandoned metal mine areas in Korea. Environ Monit Assess 2014;186(8):5209–20. 10.1007/s10661-014-3770-1. 24744211.ArticlePubMedPDF
- 16. Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, Reich A, et al. The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol 2006;1:22. 10.1186/1745-6673-1-22. 16961932.ArticlePubMedPMCPDF
- 17. Weaver VM, Kim NS, Jaar BG, Schwartz BS, Parsons PJ, Steuerwald AJ, et al. Associations of low-level urine cadmium with kidney function in lead workers. Occup Environ Med 2011;68(4):250–6. 10.1136/oem.2010.056077. 20974743.ArticlePubMedPMC
- 18. Osada M, Izuno T, Kobayashi M, Sugita M. Relationship between environmental exposure to cadmium and bone metabolism in a non-polluted area of Japan. Environ Health Prev Med 2011;16(6):341–9. 10.1007/s12199-010-0204-8. 21431812.ArticlePubMedPMCPDF
- 19. Chaumont A, Voisin C, Deumer G, Haufroid V, Annesi-Maesano I, Roels H, et al. Associations of urinary cadmium with age and urinary proteins: further evidence of physiological variations unrelated to metal accumulation and toxicity. Environ Health Perspect 2013;121(9):1047–53. 23774576.ArticlePubMedPMC
- 20. Olsson IM, Bensryd I, Lundh T, Ottosson H, Skerfving S, Oskarsson A. Cadmium in blood and urine--impact of sex, age, dietary intake, iron status, and former smoking--association of renal effects. Environ Health Perspect 2002;110(12):1185–90. 10.1289/ehp.021101185. 12460796.ArticlePubMedPMC
- 21. Lee HY, Won JC, Kang YJ, Yoon SH, Choi EO, Bae JY, et al. Type 2 diabetes in urban and rural districts in Korea: factors associated with prevalence difference. J Korean Med Sci 2010;25(12):1777–83. 10.3346/jkms.2010.25.12.1777. 21165294.ArticlePubMedPMC
- 22. Dunstan DW, Zimmet PZ, Welborn TA, De Courten MP, Cameron AJ, Sicree RA, et al. The rising prevalence of diabetes and impaired glucose tolerance: the Australian Diabetes Obesity and Lifestyle Study. Diabetes Care 2002;25(5):829–34. 10.2337/diacare.25.5.829. 11978676.PubMed
- 23. McDermott R, Rowley KG, Lee AJ, Knight S, O’Dea K. Increase in prevalence of obesity and diabetes and decrease in plasma cholesterol in a central Australian aboriginal community. Med J Aust 2000;172(10):480–4. 10901770.ArticlePubMedPDF
- 24. Afridi HI, Kazi TG, Kazi N, Jamali MK, Arain MB, Jalbani N, et al. Evaluation of status of toxic metals in biological samples of diabetes mellitus patients. Diabetes Res Clin Pract 2008;80(2):280–8. 10.1016/j.diabres.2007.12.021. 18276029.ArticlePubMed
- 25. Barregard L, Bergstrom G, Fagerberg B. Cadmium exposure in relation to insulin production, insulin sensitivity and type 2 diabetes: a cross-sectional and prospective study in women. Environ Res 2013;121:104–9. 10.1016/j.envres.2012.11.005. 23261793.ArticlePubMed
- 26. Swaddiwudhipong W, Mahasakpan P, Limpatanachote P, Krintratun S. Correlations of urinary cadmium with hypertension and diabetes in persons living in cadmium-contaminated villages in northwestern Thailand: A population study. Environ Res 2010;110(6):612–6. 10.1016/j.envres.2010.06.002. 20561611.ArticlePubMed
- 27. Nilsson T, Rorsman F, Berggren PO, Hellman B. Accumulation of cadmium in pancreatic beta cells is similar to that of calcium in being stimulated by both glucose and high potassium. Biochim Biophys Acta 1986;888(3):270–7. 10.1016/0167-4889(86)90225-9. 3530337.PubMed
- 28. Demir H, Kanter M, Coskun O, Uz YH, Koc A, Yildiz A. Effect of black cumin (Nigella sativa) on heart rate, some hematological values, and pancreatic beta-cell damage in cadmium-treated rats. Biol Trace Elem Res 2006;110(2):151–62. 10.1385/BTER:110:2:151. 16757843.PubMed
- 29. Kurata Y, Katsuta O, Doi T, Kawasuso T, Hiratsuka H, Tsuchitani M, et al. Chronic cadmium treatment induces islet B cell injury in ovariectomized cynomolgus monkeys. Jpn J Vet Res 2003;50(4):175–83. 12675553.PubMed
- 30. Chapatwala KD, Boykin M, Butts A, Rajanna B. Effect of intraperitoneally injected cadmium on renal and hepatic gluconeogenic enzymes in rats. Drug Chem Toxicol 1982;5(3):305–17. 10.3109/01480548209041060. 7151723.ArticlePubMed
- 31. Han JC, Park SY, Hah BG, Choi GH, Kim YK, Kwon TH, et al. Cadmium induces impaired glucose tolerance in rat by down-regulating GLUT4 expression in adipocytes. Arch Biochem Biophys 2003;413(2):213–20. 10.1016/S0003-9861(03)00120-6. 12729619.ArticlePubMed
- 32. Navas-Acien A, Tellez-Plaza M, Guallar E, Muntner P, Silbergeld E, Jaar B, et al. Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol 2009;170(9):1156–64. 10.1093/aje/kwp248. 19700501.ArticlePubMedPMC
- 33. Mi SQ, Yin P, Hu N, Li JH, Chen XR, Chen B, et al. BMI, WC, WHtR, VFI and BFI: which indictor is the most efficient screening index on type 2 diabetes in Chinese community population. BES 2013;26(6):485–91. 23816582.PubMed
REFERENCES
Figure & Data
REFERENCES
Citations

- Environmental exposures to lead, mercury, cadmium, manganese, and arsenic and obesity in Korean adults: Korean National Environmental Health Survey 2009–2017
Jeongwon Ock, Choong-Hee Park, Yoon-Hyeong Choi
Journal of Trace Elements in Medicine and Biology.2025; 92: 127771. CrossRef - Environmental Exposure to Cadmium and Lead Exacerbates Kidney Function in People with Diabetes
Soisungwan Satarug, David A. Vesey, Tanaporn Khamphaya, Donrawee Waeyeng, Supabhorn Yimthiang
Journal of Xenobiotics.2025; 15(6): 199. CrossRef - Co-exposure to environmental cadmium and arsenic leads to kidney damage even at lower concentrations
Jung-Eum Lee, Ju-Young Baek, Jung-Duck Park, Jun Young Chang, Byung-Sun Choi
Journal of Exposure Science & Environmental Epidemiology.2025;[Epub] CrossRef - Associations of metals and metal mixtures with glucose homeostasis: A combined bibliometric and epidemiological study
Kai Li, Yisen Yang, Jiaxin Zhao, Quan Zhou, Yanbing Li, Ming Yang, Yaoyu Hu, Jing Xu, Meiduo Zhao, Qun Xu
Journal of Hazardous Materials.2024; 470: 134224. CrossRef - Is Environmental Cadmium Exposure Causally Related to Diabetes and Obesity?
Soisungwan Satarug
Cells.2023; 13(1): 83. CrossRef - Environmental Cadmium Exposure and Type 2 Diabetes Mellitus Risk: An Overview of Systematic Reviews
Julia Hildebrand, Swarni Thakar, Tonya-Leah Watts, Laura Banfield, Lehana Thabane, Joseph Macri, Stephen Hill, M. Constantine Samaan
Exposure and Health.2022; 14(3): 743. CrossRef - Effects of Environmental Exposure to Cadmium and Lead on the Risks of Diabetes and Kidney Dysfunction
Supabhorn Yimthiang, Phisit Pouyfung, Tanaporn Khamphaya, Saruda Kuraeiad, Paleeratana Wongrith, David A. Vesey, Glenda C. Gobe, Soisungwan Satarug
International Journal of Environmental Research and Public Health.2022; 19(4): 2259. CrossRef - Cadmium exposure and risk of diabetes and prediabetes: A systematic review and dose-response meta-analysis
Tommaso Filippini, Lauren A. Wise, Marco Vinceti
Environment International.2022; 158: 106920. CrossRef - Mitigation of Cadmium Toxicity through Modulation of the Frontline Cellular Stress Response
Soisungwan Satarug, David A. Vesey, Glenda C. Gobe
Stresses.2022; 2(3): 355. CrossRef - A benchmark dose analysis for urinary cadmium and type 2 diabetes mellitus
Peng Shi, Huanchang Yan, Xingjun Fan, Shuhua Xi
Environmental Pollution.2021; 273: 116519. CrossRef - Environmental pollution and diabetes mellitus
Amany El-Sikaily, Mohamed Helal
World Journal of Meta-Analysis.2021; 9(3): 234. CrossRef - Cadmium exposure, fasting blood glucose changes, and type 2 diabetes mellitus: A longitudinal prospective study in China
Lili Xiao, Wei Li, Chunmei Zhu, Shijie Yang, Min Zhou, Bin Wang, Xing Wang, Dongming Wang, Jixuan Ma, Yun Zhou, Weihong Chen
Environmental Research.2021; 192: 110259. CrossRef - Associations between metabolic syndrome and four heavy metals: A systematic review and meta-analysis
Ping Xu, Aiping Liu, Fengna Li, Alexey A. Tinkov, Longjian Liu, Ji-Chang Zhou
Environmental Pollution.2021; 273: 116480. CrossRef - Adipose tissue cadmium concentrations as a potential risk factor for insulin resistance and future type 2 diabetes mellitus in GraMo adult cohort
Inmaculada Salcedo-Bellido, Celia Gómez-Peña, Francisco M. Pérez-Carrascosa, Petra Vrhovnik, Vicente Mustieles, Ruth Echeverría, Željka Fiket, Celia Pérez-Díaz, Rocío Barrios-Rodríguez, José Juan Jiménez-Moleón, Juan Pedro Arrebola
Science of The Total Environment.2021; 780: 146359. CrossRef - Cadmium Is Associated with Type 2 Diabetes in a Superfund Site Lead Smelter Community in Dallas, Texas
Bert B. Little, Robert Reilly, Brad Walsh, Giang T. Vu
International Journal of Environmental Research and Public Health.2020; 17(12): 4558. CrossRef - Advancing Global Health through Environmental and Public Health Tracking
Paolo Lauriola, Helen Crabbe, Behrooz Behbod, Fuyuen Yip, Sylvia Medina, Jan C. Semenza, Sotiris Vardoulakis, Dan Kass, Ariana Zeka, Irma Khonelidze, Matthew Ashworth, Kees de Hoogh, Xiaoming Shi, Brigit Staatsen, Lisbeth E. Knudsen, Tony Fletcher, Danny
International Journal of Environmental Research and Public Health.2020; 17(6): 1976. CrossRef - Assessment of heavy metals by ICP‐OES and their impact on insulin stimulating hormone and carbohydrate metabolizing enzymes
Shakil Saba, Muhammad Sajid Hamid Akash, Kanwal Rehman, Uzma Saleem, Fareeha Fiayyaz, Tanvir Ahmad
Clinical and Experimental Pharmacology and Physiology.2020; 47(10): 1682. CrossRef - Evaluation of the association between urinary cadmium levels below threshold limits and the risk of diabetes mellitus: a dose-response meta-analysis
Fei-Fei Guo, Zhi-Yong Hu, Bing-Yan Li, Li-Qiang Qin, Chunling Fu, Huifang Yu, Zeng-Li Zhang
Environmental Science and Pollution Research.2019; 26(19): 19272. CrossRef - Cadmium exposure induces pancreatic β-cell death via a Ca2+-triggered JNK/CHOP-related apoptotic signaling pathway
Cheng-Chin Huang, Chun-Ying Kuo, Ching-Yao Yang, Jui-Ming Liu, Ren-Jun Hsu, Kuan-I Lee, Chin-Chuan Su, Chin-Ching Wu, Ching-Ting Lin, Shing-Hwa Liu, Chun-Fa Huang
Toxicology.2019; 425: 152252. CrossRef - Roles of C-reactive protein on the association between urinary cadmium and type 2 diabetes
Lili Xiao, Yun Zhou, Jixuan Ma, Limin Cao, Chunmei Zhu, Wei Li, Dongming Wang, Lieyang Fan, Zi Ye, Weihong Chen
Environmental Pollution.2019; 255: 113341. CrossRef - Cadmium Body Burden and Gestational Diabetes Mellitus: A Prospective Study
Wenyu Liu, Bin Zhang, Zheng Huang, Xinyun Pan, Xiaomei Chen, Chen Hu, Hongxiu Liu, Yangqian Jiang, Xiaojie Sun, Yang Peng, Wei Xia, Shunqing Xu, Yuanyuan Li
Environmental Health Perspectives.2018;[Epub] CrossRef - Heavy Metal Exposure and Metabolic Syndrome: Evidence from Human and Model System Studies
Antonio Planchart, Adrian Green, Cathrine Hoyo, Carolyn J. Mattingly
Current Environmental Health Reports.2018; 5(1): 110. CrossRef - Cadmium affects blood pressure and negatively interacts with obesity: Findings from NHANES 1999–2014
Qi Wang, Sheng Wei
Science of The Total Environment.2018; 643: 270. CrossRef - Dietary Cadmium Intake and Its Effects on Kidneys
Soisungwan Satarug
Toxics.2018; 6(1): 15. CrossRef - Gender-specific differences of interaction between cadmium exposure and obesity on prediabetes in the NHANES 2007–2012 population
Fei Jiang, Xueyuan Zhi, Miao Xu, Bingyan Li, Zengli Zhang
Endocrine.2018; 61(2): 258. CrossRef - Association of urinary cadmium with risk of diabetes: a meta-analysis
Yujie Li, Yun Zhang, Weijing Wang, Yili Wu
Environmental Science and Pollution Research.2017; 24(11): 10083. CrossRef - Kidney Cadmium Toxicity, Diabetes and High Blood Pressure: The Perfect Storm
Soisungwan Satarug, David A. Vesey, Glenda C. Gobe
The Tohoku Journal of Experimental Medicine.2017; 241(1): 65. CrossRef - Current health risk assessment practice for dietary cadmium: Data from different countries
Soisungwan Satarug, David A. Vesey, Glenda C. Gobe
Food and Chemical Toxicology.2017; 106: 430. CrossRef - The role of cadmium in obesity and diabetes
Alexey A. Tinkov, Tommaso Filippini, Olga P. Ajsuvakova, Jan Aaseth, Yordanka G. Gluhcheva, Juliana M. Ivanova, Geir Bjørklund, Margarita G. Skalnaya, Eugenia R. Gatiatulina, Elizaveta V. Popova, Olga N. Nemereshina, Marco Vinceti, Anatoly V. Skalny
Science of The Total Environment.2017; 601-602: 741. CrossRef - Association between cadmium exposure and diabetes mellitus risk: a prisma-compliant systematic review and meta-analysis
Ming Wu, Jukun Song, Chen Zhu, Yadong Wang, Xinhai Yin, Guanglei Huang, Ke Zhao, Jianguo Zhu, Zhuhui Duan, Lingkai Su
Oncotarget.2017; 8(68): 113129. CrossRef
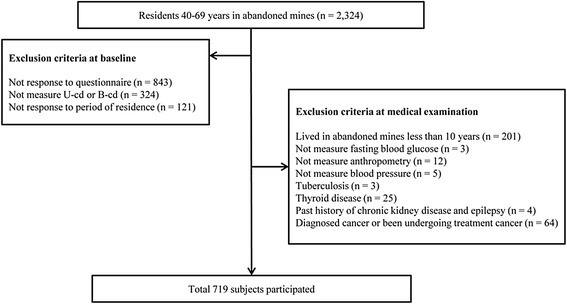
Fig. 1
| Total | Male | Female | P-value | |
|---|---|---|---|---|
| (n = 719) | (n = 489, 68.0 %) | (n = 230, 32.0 %) | ||
| Age, years | 59.1 ± 7.5 | 58.8 ± 7.5 | 59.8 ± 7.5 | 0.071 |
| BMI, kg/cm2 | 24.6 ± 3.6 | 24.2 ± 3.6 | 25.5 ± 3.5 | <0.001 |
| Systolic BP, mmHg | 133.5 ± 17.4 | 133.2 ± 17.4 | 134.1 ± 17.3 | 0.514 |
| Diastolic BP, mmHg | 79.9 ± 11.0 | 80.3 ± 11.0 | 79.1 ± 11.1 | 0.172 |
| Urine Cadmium, μg/g Cr | 2.29 ± 2.20 | 2.13 ± 2.18 | 2.63 ± 2.21 | 0.005 |
| Blood Cadmium, μg/L | 1.70 ± 0.97 | 1.73 ± 0.97 | 1.64 ± 0.95 | 0.254 |
| Glucose, mg/dL | 110.4 ± 62.0 | 114.4 ± 69.2 | 101.9 ± 42.0 | 0.003 |
| Region† | ||||
| High exposure | 267 (37.1) | 211 (43.1) | 56 (24.3) | |
| Low exposure | 452 (62.9) | 278 (56.9) | 174 (75.7) | <0.001 |
| Smoking status | ||||
| Non smoker | 247 (34.4) | 47 (9.6) | 200 (87.0) | |
| Ex-smoker | 170 (23.6) | 164 (33.5) | 6 (2.6) | |
| Current smoker | 302 (42.0) | 278 (56.9) | 24 (10.4) | <0.001 |
| Alcohol consumption | ||||
| Non-drinker | 249 (34.6) | 107 (21.9) | 142 (61.7) | |
| Ex-drinker | 96 (13.4) | 65 (13.3) | 31 (13.5) | |
| Current drinker | 374 (52.0) | 317 (64.8) | 57 (24.8) | <0.001 |
| Family income | ||||
| ≤500,000 won | 342 (47.6) | 196 (40.1) | 146 (63.5) | |
| 500,000-1,000,000 won | 147 (20.4) | 105 (21.5) | 42 (18.3) | |
| 1,000,000-1,500,000 won | 105 (14.6) | 85 (17.4) | 20 (8.7) | |
| >1,500,000 won | 125 (17.4) | 103 (21.1) | 22 (9.6) | <0.001 |
| Menopausal status | ||||
| Yes | 193 (83.9) | 193 (83.9) | ||
| No | 37 (16.1) | 37 (16.1) |
| Classification | N(%) | Urine cadmium, μg/g Cr | Blood cadmium, μg/L | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AM ± SD | p | GM(95 % CI) | p | AM ± SD | p | GM(95 % CI) | p | ||
| Age group | |||||||||
| 40–49 | 90(12.5) | 1.66 ± 1.23 | 1.55(1.38–1.74) | 1.46 ± 0.84 | 1.35(1.23–1.48) | ||||
| 50–59 | 237(33.0) | 2.18 ± 1.64 | 1.91(1.78–2.09) | 1.66 ± 0.85 | 1.51(1.45–1.62) | ||||
| 60–69 | 392(54.5) | 2.49 ± 2.61 | 0.004 | 2.04(1.91–2.14) | 0.003 | 1.79 ± 1.04 | 0.009 | 1.66(1.58–1.74) | 0.001 |
| Gender | |||||||||
| Male | 489(68.0) | 2.13 ± 2.18 | 1.82(1.74–1.91) | 1.73 ± 0.97 | 1.62(1.55–1.70) | ||||
| Female | 230(32.0) | 2.63 ± 2.21 | 0.005 | 2.14(2.00–2.34) | <0.001 | 1.64 ± 0.95 | 0.254 | 1.48(1.41–1.58) | 0.065 |
| Region | |||||||||
| High exposure | 267(37.1) | 2.52 ± 2.80 | 2.04(1.86–2.19) | 1.88 ± 0.90 | 1.74(1.66–1.82) | ||||
| Low exposure | 452(62.9) | 2.15 ± 1.75 | 0.049 | 1.86(1.74–1.95) | 0.039 | 1.60 ± 0.99 | <0.001 | 1.48(1.41–1.55) | <0.001 |
| BMI | |||||||||
| <25 | 412(57.3) | 2.37 ± 2.46 | 2.00(1.86–2.09) | 1.79 ± 1.05 | 1.62(1.55–1.70) | ||||
| ≥25 | 307(42.7) | 2.17 ± 1.81 | 0.226 | 1.86(1.70–2.00) | 0.076 | 1.59 ± 0.83 | 0.006 | 1.51(1.41–1.58) | 0.026 |
| Diabetes | |||||||||
| Non-diabetes | 561(78.0) | 2.16 ± 1.70 | 1.86(1.78–1.95) | 1.70 ± 0.95 | 1.58(1.51–1.62) | ||||
| Diabetes | 158(22.0) | 2.72 ± 3.41 | 0.049 | 2.14(1.95–2.40) | 0.009 | 1.70 ± 1.02 | 0.926 | 1.58(1.48–1.70) | 0.955 |
| Smoking | |||||||||
| Non smoker | 247(34.4) | 2.51 ± 2.82 | 2.09(1.91–2.29) | 1.52 ± 0.94 | 1.45(1.35–1.51) | ||||
| Ex-smoker | 170(27.3) | 2.10 ± 1.78 | 1.78(1.62–1.95) | 1.53 ± 0.69 | 1.45(1.38–1.55) | ||||
| Current smoker | 302(42.0) | 2.21 ± 1.81 | 0.138 | 1.91(1.78–2.04) | 0.083 | 1.95 ± 1.06 | <0.001 | 1.77(1.66–1.86) | <0.001 |
| Alcohol consumption | |||||||||
| Non-drinker | 248(34.6) | 2.57 ± 2.76 | 2.09(1.91–2.29) | 1.69 ± 0.90 | 1.55(1.45–1.62) | ||||
| Ex-drinker | 96(13.4) | 2.33 ± 1.79 | 2.04(1.78–2.29) | 1.42 ± 0.80 | 1.38(1.26–1.51) | ||||
| Current drinker | 374(52.0) | 2.09 ± 1.84 | 0.028 | 1.78(1.70–1.91) | 0.013 | 1.79 ± 1.03 | 0.004 | 1.66(1.55–1.74) | 0.009 |
| Family income (won) | |||||||||
| ≤500,000 | 342(47.6) | 2.40 ± 2.24 | 1.95(1.78–2.09) | 1.71 ± 1.00 | 1.58(1.51–1.66) | ||||
| 500,000–1,000,000 | 147(20.4) | 2.15 ± 1.56 | 1.95(1.78–2.14) | 1.84 ± 0.98 | 1.70(1.55–1.82) | ||||
| 1,000,000–1,500,000 | 105(14.6) | 2.12 ± 1.50 | 1.86(1.66–0.29) | 1.60 ± 1.00 | 1.51(1.38–1.66) | ||||
| >1,500,000 | 125(17.4) | 2.29 ± 3.09 | 0.569 | 1.91(1.74–2.14) | 0.939 | 1.62 ± 0.80 | 0.177 | 1.48(1.38–1.62) | 0.164 |
| Menopausal status ( only female) | |||||||||
| Yes | 193(83.9) | 2.70 ± 1.54 | 2.19(2.00–2.40) | 1.65 ± 0.96 | 1.51(1.41–1.62) | ||||
| No | 37(16.1) | 2.27 ± 2.32 | 0.285 | 2.04(1.66–2.51) | 0.534 | 1.59 ± 0.96 | 0.736 | 1.45(1.20–1.70) | 0.653 |
| Male(n = 466) | Female(n = 218) | |||
|---|---|---|---|---|
| Urine cadmium, μg/g creatinine | Blood cadmium, μg/L | Urine cadmium, μg/g creatinine | Blood cadmium, μg/L | |
| Age, year | 0.069 | 0.111* | 0.178** | 0.110 |
| Systolic BP, mmHg | −0.057 | −0.016 | −0.094 | −0.094 |
| Diastolic BP, mmHg | −0.020 | 0.040 | −0..080 | −0.066 |
| BMI, kg/cm2 | −0.113* | −0.178** | −0.070 | −0.074 |
| Glucose, mg/dL | 0.182** | 0.037 | −0.009 | −0.067 |
| Male(n = 489) | |||||
|---|---|---|---|---|---|
| 1sttertile | 2ndtertile | 3rdtertile | p- value | Post hoc comparison | |
| (n = 199) | (n = 151) | (n = 139) | |||
| (U-Cd ≤1 μg/g Cr) | (1 μg/g Cr < U-Cd ≤2 μg/g Cr) | (2 μg/g Cr < U-Cd) | |||
| Age, year | 57.4 ± 7.9 | 60.1 ± 7.1 | 59.1 ± 6.8 | 0.003a | I ≠ IIIb |
| Glucose, mg/dL | 105.3 ± 38.9 | 117.4 ± 66.2 | 124.3 ± 98.7 | 0.037a | I ≠ IIIb |
| BMI, kg/cm2 | 24.80 ± 4.00 | 24.09 ± 3.14 | 23.36 ± 3.29 | <0.001a | I ≠ IIIb |
| Diabetes prevalence | 17.1 % | 23.8 % | 28.8 % | 0.010 | |
| Female(n = 176) | |||||
| 1sttertile | 2ndtertile | 3rdtertile | p -value | Post hoc comparison | |
| (n = 79) | (n = 58) | (n = 39) | |||
| (U-Cd ≤1 μg/g Cr) | (1 μg/g Cr < U-Cd ≤3 μg/g Cr) | (3 μg/g Cr < U-Cd) | |||
| Age, year | 58.8 ± 8.4 | 58.8 ± 7.4 | 60.7 ± 7.0 | 0.408a | |
| Glucose, mg/dL | 102.7 ± 49.1 | 102.8 ± 41.8 | 106.0 ± 39.5 | 0.923a | |
| BMI, kg/cm2 | 25.86 ± 3.26 | 25.83 ± 3.38 | 24.48 ± 3.15 | 0.074a | |
| Diabetes prevalence | 20.3 % | 17.2 % | 30.8 % | 0.282 |
| Male (n = 489) | |||
|---|---|---|---|
| 1sttertile | 2ndtertile | 3rdtertile | |
| (n = 199) | (n = 151) | (n = 139) | |
| (U-Cd ≤1 μg/g Cr) | (1 μg/g Cr < U-Cd ≤2 μg/g Cr) | (2 μg/g Cr < U-Cd) | |
| Model 1 | Reference | 1.52 (0.90–2.57) | 1.96 (1.17–3.30) |
| Model 2 | Reference | 1.42 (0.83–2.42) | 1.89 (1.12–3.19) |
| Model 3 | Reference | 1.42 (0.83–2.45) | 1.81(1.05–3.12) |
| Female (n = 176) | |||
| 1sttertile | 2ndtertile | 3rdtertile | |
| (n = 79) | (n = 58) | (n = 39) | |
| (U–Cd ≤1 μg/g Cr) | (1 μg/g Cr < U–Cd ≤3 μg/g Cr) | (3 μg/g Cr < U–Cd) | |
| Model 1 | Reference | 0.82 (0.34–1.97) | 1.75 (0.73–4.19) |
| Model 2 | Reference | 0.82 (0.34–1.97) | 1.72 (0.72–4.15) |
| Model 3* | Reference | 0.66 (0.25–1.73) | 1.39 (0.52–3.72) |
Region was divided by kind of mines; high exposure region was Pb, Zn, Cu mines and low exposure region was otherwise mines
BMI, body mass index; BP, blood pressure; Cr, creatinine; AM, arithmetical mean; GM, geometric mean; CI, confidence interval
Region was divided by kind of mines; high exposure region was Pb, Zn, Cu mines and low exposure region was otherwise mines
BMI, body mass index; Cr, creatinine; AM, arithmetical mean; SD, standard deviation; GM, geometric mean; CI, confidence interval
*
**
aStatistical significances were tested by one way analysis of variances among groups
bThe same letters indicate non-significant difference between groups based on Tukey’s multiple comparison test
Model 1: non–adjusted
Model 2: adjusted for age
Model 3: adjusted for age, BMI, smoking, alcohol consumption, region, family income
Model 3*: adjusted for age, BMI, smoking, alcohol consumption, region, family income, menopausal status
 KSOEM
KSOEM

 Cite
Cite