Articles
- Page Path
- HOME > Ann Occup Environ Med > Volume 35; 2023 > Article
- Original Article Association between hearing loss and high-sensitivity C-reactive protein: the Kangbuk Samsung Cohort Study
-
Jihoon Kim1
 , Yesung Lee2
, Yesung Lee2 , Eunhye Seo1
, Eunhye Seo1 , Daehoon Kim1
, Daehoon Kim1 , Jaehong Lee1
, Jaehong Lee1 , Youshik Jeong1
, Youshik Jeong1 , Seonghyun Kwon1
, Seonghyun Kwon1 , Jinsook Jeong1
, Jinsook Jeong1 , Woncheol Lee1
, Woncheol Lee1
-
Annals of Occupational and Environmental Medicine 2023;35:e38.
DOI: https://doi.org/10.35371/aoem.2023.35.e38
Published online: September 11, 2023
1Department of Occupational and Environmental Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
2Medical Support Division, Pyeongchang County Public Health Clinic, Pyeongchang, Korea.
- Correspondence: Woncheol Lee. Department of Occupational and Environmental Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, 29 Saemunan-ro, Jongno-gu, Seoul 03181, Korea. doctor.oem@gmail.com
Copyright © 2023 Korean Society of Occupational & Environmental Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background Hearing loss (HL) is linked to an elevated risk of cardiovascular diseases (CVDs). The pathogeneses of HL and CVD commonly involve inflammatory responses. Previous studies investigated elevated levels of inflammatory biomarkers in subjects with HL, however, their findings did not demonstrate statistical significance. In our cross-sectional and longitudinal study, we investigated the correlation between HL and increased high-sensitivity C-reactive protein (hsCRP) levels to determine how HL is associated with CVDs.
-
Methods We conducted a cross-sectional study with workers aged over 18 years who underwent health check-ups at our institution between 2012 and 2018 (n = 566,507), followed by conducting a longitudinal study of workers aged > 18 who underwent health checkups at least twice at our institution between 2012 and 2018 (n = 173,794). The definition of HL was as an average threshold of ≥ 20 dB in pure-tone air conduction at 0.5, 1.0, and 2.0 kHz in both ears. The incidence of increased hsCRP levels throughout the follow-up period was defined as a level exceeding 3 mg/L. Logistic regression and generalized estimating equations were performed to estimate the risk of increased hsCRP levels according to the occurrence of HL in groups stratified by age.
-
Results In the cross-sectional study, the multivariate-adjusted odds ratio (OR) was 1.17 (95% confidence interval [CI]: 1.02–1.34); the OR was 0.99 (95% CI: 0.80–1.22) in those under 40 and 1.28 (1.08–1.53) in those over 40. In the longitudinal study, the multivariable-adjusted OR was 1.05 (95% CI: 0.92–1.19); the OR was 1.10 (95% CI: 0.90–1.35) in those under 40 and 1.20 (1.01–1.43) in those over 40.
-
Conclusions This cross-sectional and longitudinal study identified an association between HL and increased hsCRP levels in workers aged over 40 years.
BACKGROUND
METHODS
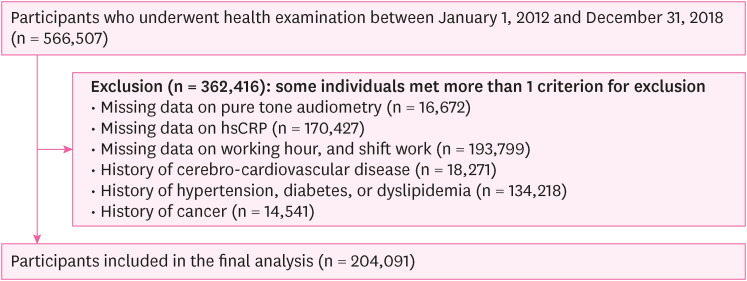
Flowchart of the study participants (cross-sectional study).
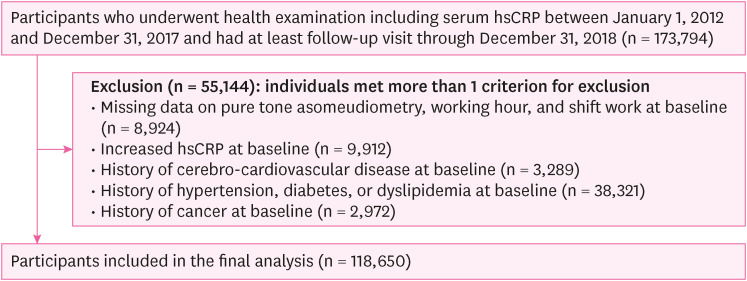
Flowchart of the study participants (longitudinal study).
RESULTS
Basic characteristics according to hearing status (cross-sectional study)
Risk of increased hsCRP according to hearing status (cross-sectional study)
Basic characteristics according to hearing status (longitudinal study)
Risk of increased hsCRP according to hearing status (longitudinal study)
DISCUSSION
CONCLUSIONS
Abbreviations
BMI
BP
CI
CRP
CVD
GEE
HL
HOMA-IR
hsCRP
IRB
KRW
LDL-C
OR
ROS
SBP
SD
-
Competing interests: The authors declare that they have no competing interests.
-
Authors contributions:
NOTES
- 1. Stevens G, Flaxman S, Brunskill E, Mascarenhas M, Mathers CD, Finucane M, et al. Global and regional hearing impairment prevalence: an analysis of 42 studies in 29 countries. Eur J Public Health 2013;23(1):146–152. 22197756.ArticlePubMed
- 2. GBD 2019 Hearing Loss Collaborators. Hearing loss prevalence and years lived with disability, 1990–2019: findings from the Global Burden of Disease Study 2019. Lancet 2021;397(10278):996–1009. 33714390.PubMedPMC
- 3. The Lancet. Hearing loss: an important global health concern. Lancet 2016;387(10036):2351. 27312288.ArticlePubMed
- 4. Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 2018;144(2):115–126. 29222544.ArticlePubMedPMC
- 5. Mener DJ, Betz J, Genther DJ, Chen D, Lin FR. Hearing loss and depression in older adults. J Am Geriatr Soc 2013;61(9):1627–1629. 24028365.ArticlePubMedPMCPDF
- 6. Chen DS, Betz J, Yaffe K, Ayonayon HN, Kritchevsky S, Martin KR, et al. Association of hearing impairment with declines in physical functioning and the risk of disability in older adults. J Gerontol A Biol Sci Med Sci 2015;70(5):654–661. 25477427.ArticlePubMedPMC
- 7. Friedland DR, Cederberg C, Tarima S. Audiometric pattern as a predictor of cardiovascular status: development of a model for assessment of risk. Laryngoscope 2009;119(3):473–486. 19235737.ArticlePubMed
- 8. Lee W, Chang Y, Shin H, Ryu S. Hearing loss and risk of overall, injury-related, and cardiovascular mortality: the Kangbuk Samsung Health Study. J Clin Med 2020;9(5):1415. 32397655.ArticlePubMedPMC
- 9. Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr 2006;83(2):456S–460S. 16470012.ArticlePubMed
- 10. Kurabi A, Keithley EM, Housley GD, Ryan AF, Wong AC. Cellular mechanisms of noise-induced hearing loss. Hear Res 2017;349:129–137. 27916698.ArticlePubMedPMC
- 11. Fischer ME, Schubert CR, Nondahl DM, Dalton DS, Huang GH, Keating BJ, et al. Subclinical atherosclerosis and increased risk of hearing impairment. Atherosclerosis 2015;238(2):344–349. 25555266.ArticlePubMedPMC
- 12. Frosolini A, Franz L, Daloiso A, Lovato A, de Filippis C, Marioni G. Digging into the role of inflammatory biomarkers in sudden sensorineural hearing loss diagnosis and prognosis: a systematic review and meta-analysis. Medicina (Kaunas) 2022;58(7):963. 35888682.ArticlePubMedPMC
- 13. Göde S, Turhal G, Kaya İ, Mavili HI, Kirazlı T. Evaluation of procalcitonin and hs-CRP levels in sudden sensorineural hearing loss. J Int Adv Otol 2018;14(1):44–47. 28639554.ArticlePubMed
- 14. Gupta S, Curhan SG, Curhan GC. Biomarkers of systemic inflammation and risk of incident hearing loss. Ear Hear 2019;40(4):981–989. 30399011.ArticlePubMedPMC
- 15. Banait T, Wanjari A, Danade V, Banait S, Jain J. Role of high-sensitivity C-reactive protein (hs-CRP) in non-communicable diseases: a review. Cureus 2022;14(10):e30225. 36381804.ArticlePubMedPMC
- 16. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003;111(12):1805–1812. 12813013.ArticlePubMedPMC
- 17. Luan YY, Yao YM. The clinical significance and potential role of C-reactive protein in chronic inflammatory and neurodegenerative diseases. Front Immunol 2018;9:1302. 29951057.ArticlePubMedPMC
- 18. Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997;336(14):973–979. 9077376.ArticlePubMed
- 19. Bassuk SS, Rifai N, Ridker PM. High-sensitivity C-reactive protein: clinical importance. Curr Probl Cardiol 2004;29(8):439–493. 15258556.ArticlePubMed
- 20. Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 2002;347(20):1557–1565. 12432042.ArticlePubMed
- 21. Chang Y, Jung HS, Cho J, Zhang Y, Yun KE, Lazo M, et al. Metabolically healthy obesity and the development of nonalcoholic fatty liver disease. Am J Gastroenterol 2016;111(8):1133–1140. 27185080.ArticlePubMedPDF
- 22. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28(7):412–419. 3899825.ArticlePubMedPDF
- 23. Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO 3rd, Criqui M, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107(3):499–511. 12551878.ArticlePubMed
- 24. Guo Y, Liu J. The roles played by blood inflammatory parameters in sudden sensorineural hearing loss. Ear Nose Throat J 2021.ArticlePDF
- 25. Masuda M, Kanzaki S, Minami S, Kikuchi J, Kanzaki J, Sato H, et al. Correlations of inflammatory biomarkers with the onset and prognosis of idiopathic sudden sensorineural hearing loss. Otol Neurotol 2012;33(7):1142–1150. 22872174.ArticlePubMed
- 26. Mattox DE, Simmons FB. Natural history of sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol 1977;86(4 Pt 1):463–480. 889223.ArticlePubMedPDF
- 27. Humes LE. The World Health Organization’s hearing-impairment grading system: an evaluation for unaided communication in age-related hearing loss. Int J Audiol 2019;58(1):12–20. 30318941.ArticlePubMedPMC
- 28. Fuentes-Santamaría V, Alvarado JC, Mellado S, Melgar-Rojas P, Gabaldón-Ull MC, Cabanes-Sanchis JJ, et al. Age-related inflammation and oxidative stress in the cochlea are exacerbated by long-term, short-duration noise stimulation. Front Aging Neurosci 2022;14:853320. 35450058.PubMedPMC
- 29. Tan WJT, Song L. Role of mitochondrial dysfunction and oxidative stress in sensorineural hearing loss. Hear Res 2023;434:108783. 37167889.ArticlePubMed
- 30. Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev 2017;2017:8416763. 28819546.ArticlePubMedPMCPDF
- 31. Marseglia L, Manti S, D’Angelo G, Nicotera A, Parisi E, Di Rosa G, et al. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci 2014;16(1):378–400. 25548896.ArticlePubMedPMC
- 32. Tan BL, Norhaizan ME, Liew WP, Sulaiman Rahman H. Antioxidant and oxidative stress: a mutual interplay in age-related diseases. Front Pharmacol 2018;9:1162. 30405405.ArticlePubMedPMC
- 33. Hajar R. Framingham contribution to cardiovascular disease. Heart Views 2016;17(2):78–81. 27512540.ArticlePubMedPMC
- 34. Watson N, Ding B, Zhu X, Frisina RD. Chronic inflammation - inflammaging - in the ageing cochlea: a novel target for future presbycusis therapy. Ageing Res Rev 2017;40:142–148. 29017893.ArticlePubMedPMC
- 35. Cunningham LL, Tucci DL. Hearing loss in adults. N Engl J Med 2017;377(25):2465–2473. 29262274.ArticlePubMedPMC
REFERENCES
REFERENCES
Figure & Data
REFERENCES
Citations

- Associations Between Inflammation and Multisensory Impairment Among Older Adults
Willa D. Brenowitz, Christina R. Sheppler, Yue Leng, Kristine Yaffe
Journal of the American Geriatrics Society.2025; 73(9): 2846. CrossRef
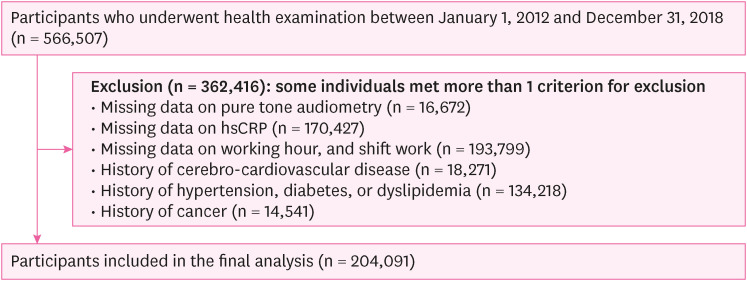
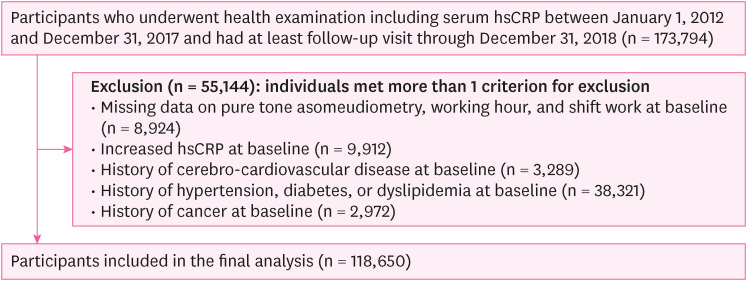
Fig. 1
Fig. 2
| Characteristics | Overall (n = 204,091) | Hearing status | ||
|---|---|---|---|---|
| Normal (n = 199,103) | Hearing loss (n = 4,988) | |||
| Sex (male) | 59.0 | 58.8 | 70.7 | < 0.001 |
| Age (years) | 35.6 ± 7.7 | 35.4 ± 7.4 | 46.5 ± 11.3 | < 0.001 |
| Age > 40 | 22.1 | 21.0 | 66.3 | < 0.001 |
| Current smoker | 20.2 | 20.0 | 26.5 | < 0.001 |
| Heavy alcohol intakea | 15.0 | 14.8 | 19.5 | < 0.001 |
| Regular exerciseb | 12.7 | 12.6 | 16.1 | < 0.001 |
| High education levelc | 82.1 | 82.6 | 63.4 | < 0.001 |
| High household incomed | 28.5 | 28.5 | 28.8 | < 0.001 |
| Marital status (married) | 70.0 | 69.6 | 84.6 | < 0.001 |
| BMI (kg/m2)e | 23.1 | 23.1 | 23.8 | < 0.001 |
| HOMA-IR | 1.22 (0.81–1.78) | 1.22 (0.81–1.78) | 1.18 (0.77–1.77) | < 0.001 |
| SBP (mmHg) | 106.8 ± 11.0 | 106.7 ± 11.0 | 109.9 ± 11.2 | < 0.001 |
| Glucose (mg/dL) | 92.4 ± 8.1 | 92.3 ± 8.1 | 95.0 ± 8.9 | < 0.001 |
| LDL-C (mg/dL) | 118.0 ± 29.9 | 117.7 ± 29.9 | 127.4 ± 31.0 | < 0.001 |
| Working hours (hours/week) | 40.7 ± 18.1 | 40.7 ± 18.0 | 40.6 ± 19.0 | < 0.001 |
| Shift workf | 12.8 | 12.8 | 9.6 | < 0.001 |
| hsCRP (mg/L) | 0.4 (0.2–0.8) | 0.4 (0.2–0.8) | 0.5 (0.3–1.0) | < 0.001 |
| hsCRPg | 5.2 | 5.2 | 6.3 | < 0.001 |
| Hearing status | OR (95% CI)a | ||||
|---|---|---|---|---|---|
| Unadjusted | Model 1b | Model 2c | Model 3d | ||
| Total | |||||
| Normal | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Hearing loss | 1.24 (1.10–1.39) | 1.25 (1.11–1.40) | 1.17 (1.02–1.34) | 1.17 (1.02–1.34) | |
| Age ≤ 40 | |||||
| Normal | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Hearing loss | 1.20 (0.98–1.46) | 1.15 (0.94–1.40) | 0.99 (0.80–1.22) | 0.99 (0.80–1.22) | |
| Age > 40 | |||||
| Normal | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Hearing loss | 1.33 (1.15–1.54) | 1.30 (1.13–1.51) | 1.28 (1.08–1.52) | 1.28 (1.08–1.53) | |
| Characteristics | Overall (n = 118,650) | Hearing status | ||
|---|---|---|---|---|
| Normal (n = 116,301) | Hearing loss (n = 2,349) | |||
| Sex (male) | 63.51 | 63.23 | 77.65 | < 0.001 |
| Age (years) | 36.2 ± 6.4 | 36.0 ± 6.3 | 42.3 ± 8.3 | < 0.001 |
| Age > 40 | 23.6 | 23.0 | 56.6 | < 0.001 |
| Current smoker | 21.05 | 20.89 | 29.12 | < 0.001 |
| Heavy alcohol intakea | 14.11 | 13.99 | 20.05 | < 0.001 |
| Regular exerciseb | 12.12 | 12.10 | 13.07 | 0.004 |
| High education levelc | 85.73 | 85.88 | 78.29 | < 0.001 |
| High household incomed | 29.80 | 29.72 | 33.80 | < 0.001 |
| Marital status (married) | 75.57 | 75.36 | 85.91 | < 0.001 |
| BMI (kg/m2)e | 23.1 | 23.1 | 23.9 | < 0.001 |
| HOMA-IR | 1.20 (0.80–1.74) | 1.20 (0.80–1.74) | 1.25 (0.84–1.85) | < 0.001 |
| SBP (mmHg) | 106.5 ± 10.9 | 106.4 ± 10.9 | 108.7 ± 10.8 | < 0.001 |
| Glucose (mg/dL) | 92.5 ± 7.9 | 92.4 ± 7.9 | 94.8 ± 8.6 | < 0.001 |
| LDL-C (mg/dL) | 117.5 ± 29.0 | 117.4 ± 29.0 | 124.1 ± 28.7 | < 0.001 |
| Working hours (hours/week) | 41.2 ± 18.5 | 41.4 ± 18.4 | 39.7 ± 19.2 | < 0.001 |
| Shift workf | 9.9 | 9.95 | 7.07 | < 0.001 |
| hsCRP (mg/L) | 0.4 (0.2–0.7) | 0.4 (0.2–0.7) | 0.5 (0.3–0.8) | < 0.001 |
| Hearing status | Incidence | OR (95% CI)a | ||||
|---|---|---|---|---|---|---|
| Unadjusted | Model 1b | Model 2c | Model 3d | |||
| Total (n = 118,650) | ||||||
| Normal (n = 116,301) | 11,049 (9.5%) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Hearing loss (n = 2,349) | 272 (11.6%) | 1.24 (1.11–1.40) | 1.08 (0.96–1.21) | 1.05 (0.92–1.19) | 1.05 (0.92–1.19) | |
| Age ≤ 40 (n = 90,633) | ||||||
| Normal (n = 89,614) | 8,211 (9.2%) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Hearing loss (n = 1,019) | 115 (11.3%) | 1.33 (1.12–1.58) | 1.28 (1.08–1.52) | 1.12 (0.92–1.36) | 1.10 (0.90–1.35) | |
| Age > 40 (n = 28,017) | ||||||
| Normal (n = 26,687) | 2,838 (10.6%) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Hearing loss (n = 1,330) | 157 (11.8%) | 1.25 (1.07–1.46) | 1.20 (1.02–1.40) | 1.19 (1.01–1.42) | 1.20 (1.01–1.43) | |
Data are expressed as the mean ± standard deviation, median (interquartile range), or percentage.
BMI: body mass index; HOMA-IR: homeostatic model assessment for insulin resistance; SBP: systolic blood pressure; LDL-C: low-density lipoprotein cholesterol; hsCRP: high-sensitivity C-reactive protein.
a≥ 30 g/day for males, ≥ 20 g/day for females; b≥ Three times/week; c≥ College graduate; dTotal monthly household income ≥ 6 million Korean Republic won/month; eBMI ≥ 25 kg/m
Bold indicates statistically significant results.
hsCRP: high-sensitivity C-reactive protein; OR: odds ratio; CI: confidence interval; BMI: body mass index; HOMA-IR: homeostatic model assessment for insulin resistance; SBP: systolic blood pressure; LDL-C: low-density lipoprotein cholesterol.
aEstimated from logistic regression models; bModel 1 was adjusted for age and sex; cModel 2: model 1 plus an adjustment for smoking status, alcohol intake, exercise, education level, total household income, marital status, BMI, HOMA-IR, SBP, glucose, and LDL-C; dModel 3: model 2 plus an adjustment for weekly working hours and shift work.
Data are expressed as the mean ± standard deviation, median (interquartile range), or percentage.
BMI, body mass index; HOMA-IR, homeostatic model assessment for insulin resistance; SBP, systolic blood pressure; LDL-C, low-density lipoprotein cholesterol.
a≥ 30 g/day for males, ≥ 20 g/day for females; b≥ Three times/week; c≥ College graduate; dTotal monthly household income ≥ 6 million Korean Republic won/month; eBMI ≥ 25 kg/m
Bold indicates statistically significant results.
hsCRP: high-sensitivity C-reactive protein; OR: odds ratio; CI: confidence interval; BMI: body mass index; HOMA-IR: homeostatic model assessment for insulin resistance; SBP: systolic blood pressure; LDL-C: low-density lipoprotein cholesterol.
aEstimated from the generalized estimating equation model; bModel 1 was adjusted for age and sex; cModel 2: model 1 plus an adjustment for smoking status, alcohol intake, exercise, education level, total household income, marital status, BMI, HOMA-IR, SBP, glucose, and LDL-C; dModel 3: model 2 plus an adjustment for weekly working hours and shift work.
 KSOEM
KSOEM

 Cite
Cite

