Articles
- Page Path
- HOME > Ann Occup Environ Med > Volume 35; 2023 > Article
- Original Article Organic solvent exposure for the chronic kidney disease: updated systematic review with meta-analysis
-
Chaeseong Lim1
 , Hyeoncheol Oh1,2
, Hyeoncheol Oh1,2
-
Annals of Occupational and Environmental Medicine 2023;35:e11.
DOI: https://doi.org/10.35371/aoem.2023.35.e11
Published online: May 17, 2023
1Department of Occupational and Environmental Medicine, Kosin University Gospel Hospital, Busan, Korea.
2Department of Occupational and Environmental Medicine, Kosin University College of Medicine, Kosin University Gospel Hospital, Busan, Korea.
- Correspondence: Hyeoncheol Oh. Department of Occupational and Environmental Medicine, Kosin University Gospel Hospital and Kosin University College of Medicine, 262 Gamcheon-ro, Seo-gu, Busan 49267, Korea. jin-hc@hanmail.net
Copyright © 2023 Korean Society of Occupational & Environmental Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background Studies on the relationship between organic solvent exposure and chronic kidney disease (CKD) have presented inconsistent results. Definition of CKD has changed in 2012, and other cohort studies have been newly published. Therefore, this study aimed to newly confirm the relationship between organic solvent exposure and CKD through an updated meta-analysis including additional studies.
-
Methods This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The search was conducted on January 2, 2023 using Embase and MEDLINE databases. Case-control and cohort studies on the relationship between organic solvent exposure and CKD were included. Two authors independently reviewed full-text.
-
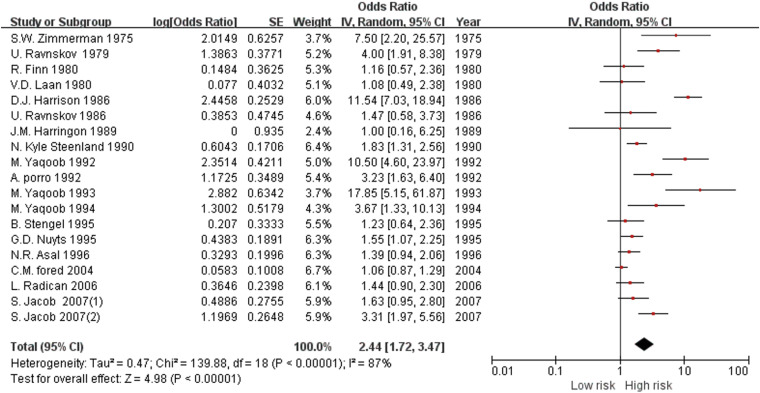
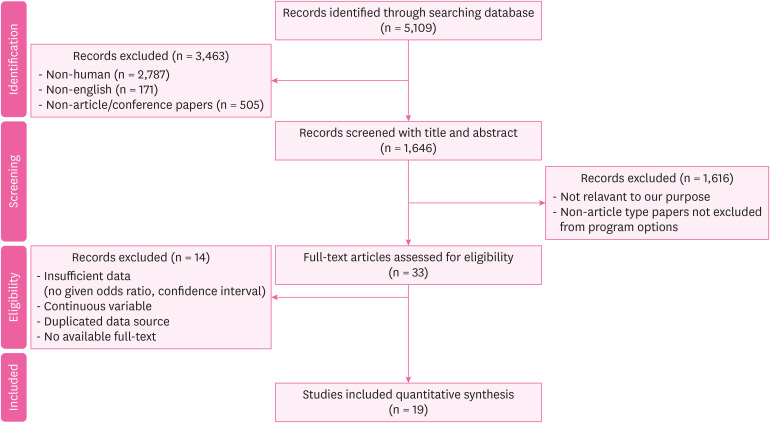
Results Of 5,109 studies identified, a total of 19 studies (control studies: 14 and cohort studies: 5) were finally included in our meta-analysis. The pooled risk of CKD in the organic solvent exposed group was 2.44 (1.72–3.47). The risk of a low-level exposure group was 1.07 (0.77–1.49). The total risk of a high-level exposure group was 2.44 (1.19–5.00). The risk of glomerulonephritis was 2.69 (1.18–6.11). The risk was 1.46 (1.29–1.64) for worsening of renal function. The pooled risk was 2.41 (1.57–3.70) in case-control studies and 2.51 (1.34–4.70) in cohort studies. The risk of subgroup classified as ‘good’ by the Newcastle Ottawa scale score was 1.93 (1.43–2.61).
-
Conclusions This study confirmed that the risk of CKD was significantly increased in workers exposed to mixed organic solvents. Further research is needed to determine the exact mechanisms and thresholds. Surveillance for kidney damage in the group exposed to high levels of organic solvents should be conducted.
-
Trial Registration PROSPERO Identifier: CRD42022306521
BACKGROUND
METHODS
Definition and criteria for CKD (KDIGO guideline11)
RESULTS
Characteristics of included studies that reported exposure to organic solvents
| Author(s) | Time of study | Study design | Data source | Number of samples | Exposure | Outcome |
|---|---|---|---|---|---|---|
| Zimmerman3 | 1975 | Case-control | Nephrology program of University of Wisconsin Center for Health Sciences, and Veterans’ Administration Hospital, Madison, Wisconsin 53706, USA | Case 63, Control 63 | Hydrocarbona | ESRD |
| Ravnskov et al.4 | 1979 | Case-control | Department of Nephrology in Lund, Sweden (otherwise, not specified) | Case 50, Control 100 | Organic solvents | GN |
| Finn et al.18 | 1980 | Case-control | Hospital inpatients (otherwise, not specified) | Case 89, Control 53 | Hydrocarbona | ESRD |
| van der Laan5 | 1980 | Case-control | Renal Pathology Department of the University of Amsterdam (otherwise, not specified) | Case 50, Control 50 | Organic solvents | GN |
| Harrison et al.19 | 1986 | Case-control | Patients who presented with membranous GN in the Edinburgh region over 20 years (otherwise, not specified) | Case 65, Control 1,746,530 | Organic solvents | GN |
| Harrington et al.8 | 1989 | Case-control | Referents: west midlands, community-based referents renal cancer case: drawn from names of all living patients with histologically proved renal adenocarcinoma (clear cell) diagnosed from May 1984 to April 1985 and recorded in the west midland’s regional cancer registry (contacted by post, participate after agreement) | Case 50, Control 50 | Organic solvents | GN, renal adenocarcinoma |
| GN case: patients attending for renal biopsy at the Queen Elizabeth Hospital, Birmingham | ||||||
| Steenland et al.20 | 1990 | Case-control | Edinburgh Renal Clinics (otherwise, not specified) | Case 325, Control 325 | Oragnic solvents | ESRD |
| Porro et al.21 | 1992 | Case-control | Case: clinical records of the Nephrology Department at the University of Bari | Case 60, Control 120 | Organic solvents | GN |
| Control: outpatients at the University of Bari | ||||||
| Yaqoob et al.22 | 1992 | Case-control | Case: Patients undergoing regular dialysis therapy between January 1988 and December 1989 and supervised by the Mersey Regional Renal Unit | Case 55 Control 55 | Hydrocarbona | ESRD |
| Control: normal controls attending a surgical day unit for minor surgical procedures | ||||||
| Yaqoob et al.26 | 1994 | Case-control | Patients with type 1 diabetes of over 10 years duration who regularly attended diabetic and renal clinics over a 6-month period were assessed. | Case 68, Control 45 | Hydrocarbona | Diabetic nephropathy |
| Stengel et al.23 | 1995 | Case-control | Cases were identified from the list of all patients whose GN was first diagnosed between January 1985 and December 1990 in Pathology Departments of 5 hospitals in Paris region. | Case 298, Control 298 | Organic solvents | GN |
| Nuyts et al.24 b | 1995 | Case-control | Cases were recruited from five renal units in three industrial areas (antwerp, Liege, and Turnhout). | Case 272, Control 272 | Copperb, Chromium, Tin, Mercury, Welding Fumes, Silicon, Grain dust, Hydrocarbona | Chronic renal failure (defined as a consistent calculated creatinine clearance under the third percentile of the normal distribution) |
| Controls were randomly selected from lists of voters in Antwerp, Turnhout, and Liege and in three rural communities (Brasschaat, Gierle, and Neupre) from the same catchment areas. | ||||||
| Asal et al.25 | 1996 | Case-control | cases were obtained from hospitals in metropolitan areas of Oklahoma City and Tulsa, Oklahoma. All major hospitals and nephrology clinics were contacted. Participating hospitals, nephrology clinics, and nephrologists were requested to provide a complete list of their patients whose diagnoses had an ICD code pertinent to chronic renal disease between January 1, 1985 and December 31, 1992. | Case 321, Control 321 | Hydrocarbona | GN |
| Fored et al.9 | 2004 | Case-control | The continuously updated Swedish National Population Register provided a well-defined study base of all 5.3 million native Swedes aged 18 to 74 years who were residents in the country during the ascertainment period (May 20, 1996 through May 31, 1998). | Case 913, Control 991 | Organic solvents | Chronic renal failure (men and women whose serum creatinine level exceeded 300 μmol/L (3.4 mg/dL) and 250 μmol/L (2.8 mg/dL)) |
| Ravnskov27 | 1986 | Cohort (prospective) | Department of Nephrology of the University Hospital Lund, Sweden (otherwise, not specified) | 71 | Hydrocarbona | Chronic renal failure (GFR < 80 mL/min) |
| Yaqoob et al.7 | 1993 | Cohort (retrospective) | Patients with biopsy-proven GN of duration > 1 year (proliferative, n = 60; membranous, n = 8) who regularly attended renal clinics over six months during 1989. | 68 | Hydrocarbona | Progressive renal failure (defined as persistent rise of serum creatinine > 50 μmol/L above the baseline) |
| Radican et al.28 | 2006 | Cohort (retrospective) | Data from 3 sources: a database of former civilian employees of the Hill Air Force Base in Utah, mortality data from the National Death Index (NDI), and ESRD incidence data from the U.S. Renal Data System (USRDS) database | 14,455 | Hydrocarbona | ESRD |
| Jacob et al.29 | 2007 | Cohort (retrospective) | GN-PROGRESS retrospective cohort study in 11 nephrology departments of the Paris region (see acknowledgments for a list of participating centers) | 338 | Organic solvents | ESRD |
| Jacob et al.30 | 2007 | Cohort (retrospective) | GN-PROGRESS retrospective cohort study. All new Caucasian adult patients with biopsy proven IgA nephropathy (IgAN), membranous nephropathy, and focal and segmental glomerulosclerosis diagnosed between January 1994 and June 2001 in 11 nephrology departments in the Paris area were invited to participate in the study between January 2002 and March 2004. | 269 | Organic solvents | ESRD |
Overall effect of organic solvents
Forest plot of the overall risk of chronic kidney disease for exposure to organic solvents.
Subgroup analysis according to exposure level
Subgroup analysis according to exposure level, disease category, study design, and quality of study
Subgroup analysis according to disease category
Subgroup analysis according to study design
Subgroup analysis according to NOS score
Quality assessment of studies included in the systematic review according to the Newcastle Ottawa Scale
| Study (cohort) | Year | Selection representativeness of the sample | Selection of the non-intervention cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability based on design and analysis | Outcome assessment of outcome | Was follow up long enough for outcomes to occur | Adequacy of follow up of cohorts | Total score | Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ravnskov27 | 1986 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 5 | Poor |
| Yaqoob et al.7 | 1993 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | Good |
| Radican et al.28 | 2006 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | Good |
| Jacob et al.29 | 2007 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 7 | Good |
| Jacob et al.30 | 2007 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 7 | Good |
| Study (case-control) | Selection is the case definition adequate? | Representativeness of cases | Selection of controls | Definition of controls | Comparability based on design and analysis | Outcome assessment of exposure | Same method of ascertainment for cases and controls | Non-response rate | Total score | Assessment | |
| Zimmerman3 | 1975 | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 0 | 6 | Poor |
| Ravnskov et al.4 | 1979 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 7 | Good |
| Finn et al.18 | 1980 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 4 | Poor |
| van der Laan5 | 1980 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 0 | 5 | Poor |
| Harrison et al.19 | 1986 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | Poor |
| Harrington et al.8 | 1989 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 | Good |
| Steenland et al.20 | 1990 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 | Good |
| Yaqoob et al.22 | 1992 | 1 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 6 | Fair |
| Porro et al.21 | 1992 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 7 | Good |
| Yaqoob et al.26 | 1994 | 1 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 6 | Fair |
| Stengel et al.23 | 1995 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | Poor |
| Nuyts et al.24 | 1995 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 7 | Good |
| Asal et al.25 | 1996 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 7 | Good |
| Fored et al.9 | 2004 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 7 | Good |
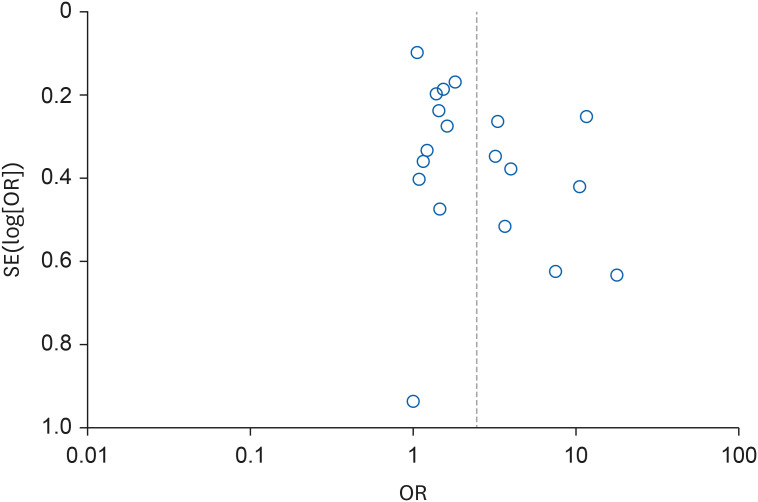
Funnel plot for overall risk of chronic kidney disease according to organic solvent exposure.
GRADE approach for the primary outcome
DISCUSSION
Definition of high vs. low exposure in each studies
| Studies | Definition of high vs. low exposure | |
|---|---|---|
| Ravnskov4 (1979) | Intensity factor | |
| 2 = occupational house painting indoor, industrial spray-painting without protection devices, carpet and floor laying, production of paint and glue, polyester plastic application | ||
| 1 = non-occupational house painting indoor, spray-painting with protection devices, industrial degreasing of metal, printing work, occupational gluing, anesthesiologic work, dry cleaning | ||
| 0.5 = outdoor painting, motor repairing, handling of petrol, hobby gluing, drawing with filter-tipped pens | ||
| Intensity: Hour of exposure × Year of exposure × Intensity factor | ||
| 50 ≤ high exposed | ||
| 10 ≤ and < 50 exposed | ||
| < 10 non-exposed | ||
| Harrington et al.8 (1989) | Exposure indices (EIs) were computed for each relevant solvent type up to the time of diagnosis or retirement, whichever was the earlier. EI were then calculated by multiplying the score by the total duration of exposure, adjusted so that 1-year full time heavy exposure corresponded to an exposure index of 100. An overall EI for “total solvent” exposures was obtained simply by summing the EIs for individual solvents. | |
| 100 ≤ EI: Exposed | ||
| 1 ≤ EI < 100: Intermediate | ||
| EI < 1: Non-exposed | ||
| Porro et al.21 (1992) | Intensity factor | |
| 2 = production of paint and glue, occupational painting indoors, spray painting without protection devices, polyester resin application with heavy contact with styrene, tank cleaning without protection devices, carpet cleaning | ||
| 1 = non-occupational painting indoors, spray painting with protection devices, industrial degreasing of metals, printing work(rotogravure), occupational gluing(including vamps gluing), dry cleaning, anesthetic work, occupational use of hair sprays, use of pesticides, polyester resin application with low exposure to styrene | ||
| 0.5 = outdoor painting, motor repairing, hobby gluing, drawing with felt tipped pens, exposure to exhaust fumes outdoors, handling of petrol fuels, degreasing of metals(excluding industrial degreasing) | ||
| Intensity: Hour of exposure × Year of exposure × Intensity factor | ||
| High exposure = Above median | ||
| Lower exposure = Below median | ||
| No exposure = Reference | ||
| (median: occupational 80.12, non-occupational 2.76) | ||
| Stengel et al.23 (1995) | Two industrial hygiene experts assessed exposure to 30 different types of solvents as well as the level (low, medium, high) and the frequency of exposure (i.e., once a week, subdivided as < 2 h/wk, 2–20 h/wk, and > 20 h/wk) (The examples of method to calculate exposure level are not provided). | |
| No exposure: No exposure | ||
| Low exposure: Defined as < 2 h/wk whatever the level or low level whatever the frequency (< 2 h/wk or low level in the tables) | ||
| Fored et al.9 (2004) | The exposure was classified on a 5-level scale in terms of approximated additive HE. Exposure to an HE of 1.0 for a single solvent corresponds to an average exposure level during an 8-hour working day equal to the OEL prescribed by the Swedish Work Environment Authority in 1996 (Ordinance on Occupational Exposure Limit Values, ASF 1996:2 [In Swedish], Stockholm, National Board of Occupational Safety and Health [Swedish Work Environment Authority], 1996) | |
| Cumulative lifetime exposure for organic solvents was calculated as the product of the intensity (HE), exposure frequency (days per month), and the duration (years) of the exposure, summed over all work periods in the subject’s occupational history. | ||
| Lifetime cumulative dose | ||
| First-Second quartile: Low exposure | ||
| Third-Fourth quartile: High exposure | ||
| Jacob et al. 29 (2007) | Job periods were reviewed, and solvent exposure level (low, medium, high) and frequency (occasional [i.e., less than once a week] or regular [i.e., once a week], subdivided as < 2, 2 to 20, and > 20 h/wk) was determined by 2 industrial hygienists. (The examples of method to calculate exposure level are not provided) | |
| No exposure = No exposure | ||
| Low exposure = Low intensity or frequency < 2 h/wk. | ||
| High exposure = High intensity and frequency ≥ 2 h/wk. | ||
CONCLUSIONS
Abbreviations
ACR
CI
CKD
ESRD
GFR
GN
GRADE
HE
ICD
IV
KDIGO
MIBK
NOS
OEL
OR
PECO
PRISMA
RBD
SE
WBC
-
Competing interests: The authors declare that they have no competing interests.
-
Author Contributions:
NOTES
- 1. Andersen M, MacNaughton M, Clewell HJ. Threshold limit values for chemical substances and physical agents and biological exposure indices. Biological Exposure Indices. 1998 TLVs and BEIs. Cincinnati, OH, USA: American Conference of Governmental Industrial Hygienists (ACGIH); 1998, 541–550.
- 2. Korea Occupational Safety and Health Agency. Korea Occupational Safety and Health Research Institute. Evaluation of the Strike and Multiple Organic Solvents. Ulsan, Korea: Korea Occupational Safety and Health Agency; 2003.
- 3. Zimmerman SW, Groehler K, Beirne GJ. Hydrocarbon exposure and chronic glomerulonephritis. Lancet 1975;2(7927):199–201. 51959.ArticlePubMed
- 4. Ravnskov U, Forsberg B, Skerfving S. Glomerulonephritis and exposure to organic solvents. A case-control study. Acta Med Scand 1979;205(7):575–579. 474184.PubMed
- 5. van der Laan G. Chronic glomerulonephritis and organic solvents. A case-control study. Int Arch Occup Environ Health 1980;47(1):1–8. 7429643.PubMed
- 6. Bell GM, Gordon AC, Lee P, Doig A, MacDonald MK, Thomson D, et al. Proliferative glomerulonephritis and exposure to organic solvents. Nephron J 1985;40(2):161–165.ArticlePDF
- 7. Yaqoob M, Stevenson A, Mason H, Bell GM. Hydrocarbon exposure and tubular damage: additional factors in the progression of renal failure in primary glomerulonephritis. Q J Med 1993;86(10):661–667. 8255964.ArticlePubMed
- 8. Harrington JM, Whitby H, Gray CN, Reid FJ, Aw TC, Waterhouse JA. Renal disease and occupational exposure to organic solvents: a case referent approach. Br J Ind Med 1989;46(9):643–650. 2789968.ArticlePubMedPMC
- 9. Fored CM, Nise G, Ejerblad E, Fryzek JP, Lindblad P, McLaughlin JK, et al. Absence of association between organic solvent exposure and risk of chronic renal failure: a nationwide population-based case-control study. J Am Soc Nephrol 2004;15(1):180–186. 14694171.PubMed
- 10. Ravnskov U. Hydrocarbon exposure may cause glomerulonephritis and worsen renal function: evidence based on Hill’s criteria for causality. QJM 2000;93(8):551–556. 10924538.ArticlePubMed
- 11. Stevens P, Levin A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013;158(11):825–830. 23732715.ArticlePubMed
- 12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021;10(1):89. 33781348.PubMedPMC
- 13. Morgan RL, Whaley P, Thayer KA, Schünemann HJ. Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int 2018;121(Pt 1):1027–1031. 30166065.ArticlePubMedPMC
- 14. Wells G, Shea B, O’Connell J. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, Canada: Ottawa Health Research Institute; 2011.
- 15. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557–560. 12958120.ArticlePubMedPMC
- 16. Hayashino Y, Noguchi Y, Fukui T. Systematic evaluation and comparison of statistical tests for publication bias. J Epidemiol 2005;15(6):235–243. 16276033.ArticlePubMedPMC
- 17. Shim SR, Shin IS, Bae JM. Intervention meta-analysis using STATA software. J Health Info Stat 2016;41(1):123–134.ArticlePDF
- 18. Finn R, Fennerty AG, Ahmad R. Hydrocarbon exposure and glomerulonephritis. Clin Nephrol 1980;14(4):173–175. 7428191.PubMed
- 19. Harrison DJ, Thomson D, MacDonald MK. Membranous glomerulonephritis. J Clin Pathol 1986;39(2):167–171. 3950037.ArticlePubMedPMC
- 20. Steenland NK, Thun MJ, Ferguson CW, Port FK. Occupational and other exposures associated with male end-stage renal disease: a case/control study. Am J Public Health 1990;80(2):153–157. 2153349.ArticlePubMedPMC
- 21. Porro A, Lomonte C, Coratelli P, Passavanti G, Ferri GM, Assennato G. Chronic glomerulonephritis and exposure to solvents: a case-referent study. Br J Ind Med 1992;49(10):738–742. 1419865.ArticlePubMedPMC
- 22. Yaqoob M, Bell GM, Percy DF, Finn R. Primary glomerulonephritis and hydrocarbon exposure: a case-control study and literature review. Q J Med 1992;83(301):409–418. 1438676.PubMed
- 23. Stengel B, Cénée S, Limasset JC, Protois JC, Marcelli A, Brochard P, et al. Organic solvent exposure may increase the risk of glomerular nephropathies with chronic renal failure. Int J Epidemiol 1995;24(2):427–434. 7635606.ArticlePubMed
- 24. Nuyts GD, Van Vlem E, Thys J, De Leersnijder D, D’Haese PC, Elseviers MM, et al. New occupational risk factors for chronic renal failure. Lancet 1995;346(8966):7–11. 7603180.ArticlePubMed
- 25. Asal NR, Cleveland HL, Kaufman C, Nsa W, Nelson DI, Nelson RY, et al. Hydrocarbon exposure and chronic renal disease. Int Arch Occup Environ Health 1996;68(4):229–235. 8738352.ArticlePubMedPDF
- 26. Yaqoob M, Patrick AW, McClelland P, Stevenson A, Mason H, Percy DF, et al. Occupational hydrocarbon exposure and diabetic nephropathy. Diabet Med 1994;11(8):789–793. 7851074.ArticlePubMed
- 27. Ravnskov U. Influence of hydrocarbon exposure on the course of glomerulonephritis. Nephron J 1986;42(2):156–160.ArticlePDF
- 28. Radican L, Wartenberg D, Rhoads GG, Schneider D, Wedeen R, Stewart P, et al. A retrospective occupational cohort study of end-stage renal disease in aircraft workers exposed to trichloroethylene and other hydrocarbons. J Occup Environ Med 2006;48(1):1–12. 16404204.ArticlePubMedPMC
- 29. Jacob S, Héry M, Protois JC, Rossert J, Stengel B. Effect of organic solvent exposure on chronic kidney disease progression: the GN-PROGRESS cohort study. J Am Soc Nephrol 2007;18(1):274–281. 17135394.PubMed
- 30. Jacob S, Héry M, Protois JC, Rossert J, Stengel B. New insight into solvent-related end-stage renal disease: occupations, products and types of solvents at risk. Occup Environ Med 2007;64(12):843–848. 17567724.ArticlePubMedPMC
- 31. Hsu CY, Chertow GM. Chronic renal confusion: insufficiency, failure, dysfunction, or disease. Am J Kidney Dis 2000;36(2):415–418. 10922323.ArticlePubMed
- 32. Evans D. Hierarchy of evidence: a framework for ranking evidence evaluating healthcare interventions. J Clin Nurs 2003;12(1):77–84. 12519253.ArticlePubMed
- 33. Mutti A, Coccini T, Alinovi R, Toubeau G, Broeckaert F, Bergamaschi E, et al. Exposure to hydrocarbons and renal disease: an experimental animal model. Ren Fail 1999;21(3-4):369–385. 10416216.ArticlePubMed
- 34. Landry JF, Langlois S. Acute exposure to aliphatic hydrocarbons: an unusual cause of acute tubular necrosis. Arch Intern Med 1998;158(16):1821–1823. 9738613.ArticlePubMed
- 35. Franchini I, Cavatorta A, Falzoi M, Lucertini S, Mutti A. Early indicators of renal damage in workers exposed to organic solvents. Int Arch Occup Environ Health 1983;52(1):1–9. 6603422.ArticlePubMedPDF
- 36. Winchester RV, Madjar VM. Solvent effects on workers in the paint, adhesive and printing industries. Ann Occup Hyg 1986;30(3):307–317. 3777750.PubMed
REFERENCES
REFERENCES
Appendix
Supplementary methods
Figure & Data
REFERENCES
Citations

- Environmental Pollution and Its Impact on Kidney Diseases: A Comprehensive Review of Current Evidence
Seung Eun Lee, Yong Seek Park
Life.2026; 16(2): 291. CrossRef - Identifying and Prioritizing Hazardous Chemicals in Construction Metal Structure Coating Systems: A Roadmap for Data‐Driven Disease Prevention
Paridhi Patel, Dhimiter Bello, Anila Bello
American Journal of Industrial Medicine.2025;[Epub] CrossRef - Mixed Solvency Concept to Replace Harmful Organic Solvent: Recent Trends and Future Challenges in Formulation Development
Pranjal Kumar Singh, Nidhi Singh, Atul Pratap Singh, Poonam Bhardwaj, Kapil Sachan, Smita Singh
Combinatorial Chemistry & High Throughput Screening.2025; 28(2): 226. CrossRef - The Exposome and the Kidney: A Silent Dialogue Shaping Chronic Kidney Disease
Livia Alvarenga, Marcia Ribeiro, Ludmila F. M. F. Cardozo, Natália A. Borges, Peter Stenvinkel, Denise Mafra
Journal of Xenobiotics.2025; 15(3): 73. CrossRef - Association between job characteristics and kidney function among men: a cross-sectional analysis of the Rafsanjan cohort study
Zahra Jamali, Reza Hosseiniara, Parvin Khalili, Fatemeh Ayoobi, Sadegh Zarei, Zahra Ahmadi, Seyed Mojtaba Heydari Khoormizi, Alireza Nazari
BMC Nephrology.2025;[Epub] CrossRef
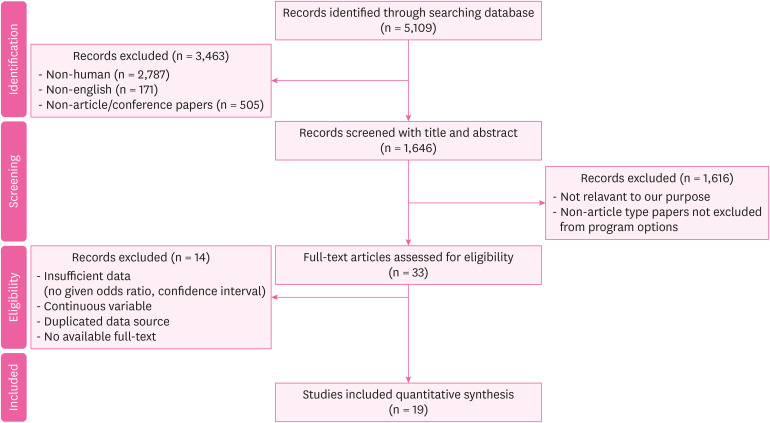
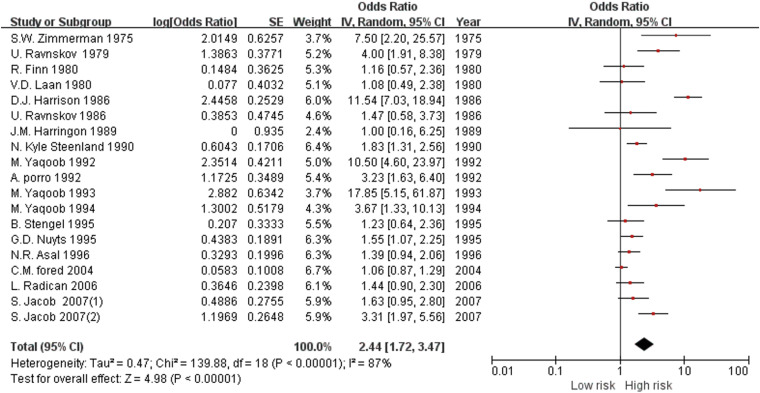
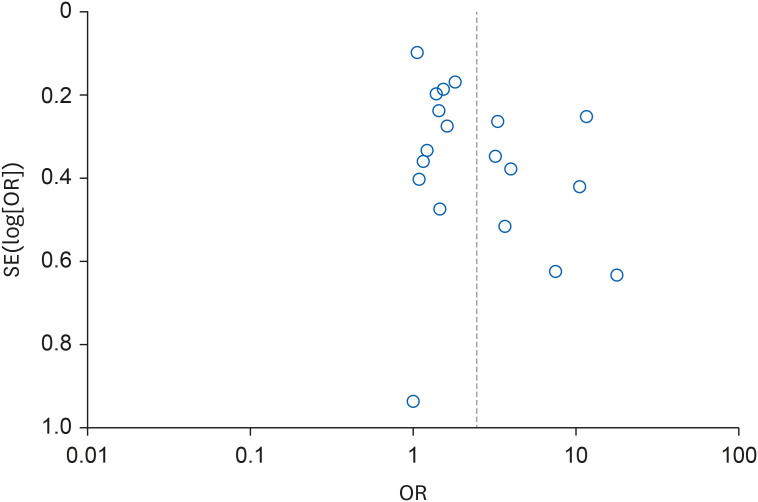
Fig. 1
Fig. 2
Fig. 3
| Definition | ||
|---|---|---|
| CKD is defined based on the presence of either kidney damage or decreased kidney function for three or more months, irrespective of cause. | ||
| Criteria | Comment | |
| Duration ≥ 3 months, based on documentation of inference | Duration is necessary to distinguish chronic from acute kidney diseases. | |
| - Clinical evaluation can often suggest duration | ||
| - Documentation of duration is usually not available in epidemiologic studies | ||
| GFR < 60 mL/min/1.73 m2 | GFR is the best overall index of kidney function in health and disease. | |
| - The normal GFR in young adults is approximately 125 mL/min/1.73 m2; GFR < 15 mL/min/1.73 m2 is defined as kidney failure | ||
| - Decreased GFR can be detected by current estimating equations for GFR based on serum creatinine (estimated GFR) but not by serum creatinine alone | ||
| - Decreased estimated GFR can be confirmed by measured GFR, measured creatinine clearance, or estimated GFR using cystatin C | ||
| Kidney damage, as defined by structural abnormalities or functional abnormalities other than decreased GFR | Pathologic abnormalities (examples). Cause is based on underlying illness and pathology. Markers of kidney damage may reflect pathology. | |
| - Glomerular diseases (diabetes, autoimmune diseases, systemic infections, drugs, neoplasia) | ||
| - Vascular diseases (atherosclerosis, hypertension, ischemia, vasculitis, thrombotic microangiopathy) | ||
| - Tubulointestitial diseases (urinary tract infections, stones, obstruction, drug toxicity) | ||
| - Cystic disease (polycystic kidney disease) | ||
| History of kidney transplantation. | ||
| - Chronic allograft nephropathy (non-specific findings of tubular atrophy, interstitial fibrosis, vascular and glomerular sclerosis) | ||
| - Rejection | ||
| - Drug toxicity (calcineurin inhibitors) | ||
| - BK virus nephropathy | ||
| - Recurrent disease (glomerular disease, oxalosis, Fabry disease) | ||
| Albuminuria as marker of kidney damage (increased glomerular permeability, urine ACR > 30 mg/g)a. | ||
| - The normal urine ACR in young adults is < 10 mg/g. Urine ACR categories 10–29, 30–300 and > 300 mg are termed “mildly increased, moderately increased, and severely increased” respectively. Urine ACR > 2,200 mg/g is accompanied by signs and symptoms of nephrotic syndrome (low serum albumin, edema, and high serum cholesterol). | ||
| - Threshold value corresponds approximately to urine dipstick values of trace or 1+, depending on urine concentration | ||
| - High urine ACR can be confirmed by urine albumin excretion in a timed urine collection | ||
| Urinary sediment abnormalities as markers of kidney damage, for example: | ||
| - RBC casts in proliferative glomerulonephritis | ||
| - WBC casts in pyelonephritis or interstitial nephritis | ||
| - Granular casts and renal tubular epithelial cells in many parenchymal diseases (non-specific) | ||
| Imaging abnormalities as markers of kidney damage (ultrasound, computed tomography and magnetic resonance imaging with or without contrast, isotope scans, angiography) | ||
| - Polycystic kidneys | ||
| - Hydronephrosis due to obstruction | ||
| - Cortical scarring due to infarcts, pyelonephritis or vesicoureteral reflux | ||
| - Renal masses or enlarged kidneys due to infiltrative disease | ||
| - Renal artery stenosis | ||
| - Small and echogenic kidneys (common in later stages of CKD due to many parenchymal diseases) | ||
| Author(s) | Time of study | Study design | Data source | Number of samples | Exposure | Outcome |
|---|---|---|---|---|---|---|
| Zimmerman | 1975 | Case-control | Nephrology program of University of Wisconsin Center for Health Sciences, and Veterans’ Administration Hospital, Madison, Wisconsin 53706, USA | Case 63, Control 63 | Hydrocarbona | ESRD |
| Ravnskov et al. | 1979 | Case-control | Department of Nephrology in Lund, Sweden (otherwise, not specified) | Case 50, Control 100 | Organic solvents | GN |
| Finn et al. | 1980 | Case-control | Hospital inpatients (otherwise, not specified) | Case 89, Control 53 | Hydrocarbona | ESRD |
| van der Laan | 1980 | Case-control | Renal Pathology Department of the University of Amsterdam (otherwise, not specified) | Case 50, Control 50 | Organic solvents | GN |
| Harrison et al. | 1986 | Case-control | Patients who presented with membranous GN in the Edinburgh region over 20 years (otherwise, not specified) | Case 65, Control 1,746,530 | Organic solvents | GN |
| Harrington et al. | 1989 | Case-control | Referents: west midlands, community-based referents renal cancer case: drawn from names of all living patients with histologically proved renal adenocarcinoma (clear cell) diagnosed from May 1984 to April 1985 and recorded in the west midland’s regional cancer registry (contacted by post, participate after agreement) | Case 50, Control 50 | Organic solvents | GN, renal adenocarcinoma |
| GN case: patients attending for renal biopsy at the Queen Elizabeth Hospital, Birmingham | ||||||
| Steenland et al. | 1990 | Case-control | Edinburgh Renal Clinics (otherwise, not specified) | Case 325, Control 325 | Oragnic solvents | ESRD |
| Porro et al. | 1992 | Case-control | Case: clinical records of the Nephrology Department at the University of Bari | Case 60, Control 120 | Organic solvents | GN |
| Control: outpatients at the University of Bari | ||||||
| Yaqoob et al. | 1992 | Case-control | Case: Patients undergoing regular dialysis therapy between January 1988 and December 1989 and supervised by the Mersey Regional Renal Unit | Case 55 Control 55 | Hydrocarbona | ESRD |
| Control: normal controls attending a surgical day unit for minor surgical procedures | ||||||
| Yaqoob et al. | 1994 | Case-control | Patients with type 1 diabetes of over 10 years duration who regularly attended diabetic and renal clinics over a 6-month period were assessed. | Case 68, Control 45 | Hydrocarbona | Diabetic nephropathy |
| Stengel et al. | 1995 | Case-control | Cases were identified from the list of all patients whose GN was first diagnosed between January 1985 and December 1990 in Pathology Departments of 5 hospitals in Paris region. | Case 298, Control 298 | Organic solvents | GN |
| Nuyts et al. | 1995 | Case-control | Cases were recruited from five renal units in three industrial areas (antwerp, Liege, and Turnhout). | Case 272, Control 272 | Copperb, Chromium, Tin, Mercury, Welding Fumes, Silicon, Grain dust, Hydrocarbona | Chronic renal failure (defined as a consistent calculated creatinine clearance under the third percentile of the normal distribution) |
| Controls were randomly selected from lists of voters in Antwerp, Turnhout, and Liege and in three rural communities (Brasschaat, Gierle, and Neupre) from the same catchment areas. | ||||||
| Asal et al. | 1996 | Case-control | cases were obtained from hospitals in metropolitan areas of Oklahoma City and Tulsa, Oklahoma. All major hospitals and nephrology clinics were contacted. Participating hospitals, nephrology clinics, and nephrologists were requested to provide a complete list of their patients whose diagnoses had an ICD code pertinent to chronic renal disease between January 1, 1985 and December 31, 1992. | Case 321, Control 321 | Hydrocarbona | GN |
| Fored et al. | 2004 | Case-control | The continuously updated Swedish National Population Register provided a well-defined study base of all 5.3 million native Swedes aged 18 to 74 years who were residents in the country during the ascertainment period (May 20, 1996 through May 31, 1998). | Case 913, Control 991 | Organic solvents | Chronic renal failure (men and women whose serum creatinine level exceeded 300 μmol/L (3.4 mg/dL) and 250 μmol/L (2.8 mg/dL)) |
| Ravnskov | 1986 | Cohort (prospective) | Department of Nephrology of the University Hospital Lund, Sweden (otherwise, not specified) | 71 | Hydrocarbona | Chronic renal failure (GFR < 80 mL/min) |
| Yaqoob et al. | 1993 | Cohort (retrospective) | Patients with biopsy-proven GN of duration > 1 year (proliferative, n = 60; membranous, n = 8) who regularly attended renal clinics over six months during 1989. | 68 | Hydrocarbona | Progressive renal failure (defined as persistent rise of serum creatinine > 50 μmol/L above the baseline) |
| Radican et al. | 2006 | Cohort (retrospective) | Data from 3 sources: a database of former civilian employees of the Hill Air Force Base in Utah, mortality data from the National Death Index (NDI), and ESRD incidence data from the U.S. Renal Data System (USRDS) database | 14,455 | Hydrocarbona | ESRD |
| Jacob et al. | 2007 | Cohort (retrospective) | GN-PROGRESS retrospective cohort study in 11 nephrology departments of the Paris region (see acknowledgments for a list of participating centers) | 338 | Organic solvents | ESRD |
| Jacob et al. | 2007 | Cohort (retrospective) | GN-PROGRESS retrospective cohort study. All new Caucasian adult patients with biopsy proven IgA nephropathy (IgAN), membranous nephropathy, and focal and segmental glomerulosclerosis diagnosed between January 1994 and June 2001 in 11 nephrology departments in the Paris area were invited to participate in the study between January 2002 and March 2004. | 269 | Organic solvents | ESRD |
| Categories | Number of results | OR (95% CI) | |
|---|---|---|---|
| Exposure level | |||
| High exposure | 6 | 2.44 (1.19–5.00) | |
| Low exposure | 6 | 1.07 (0.77–1.49) | |
| Disease category | |||
| GN | 6 | 2.69 (1.18–6.11) | |
| Worsening of renal function | 6 | 1.46 (1.29–1.64) | |
| Study design | |||
| Case-control | 12 | 2.41 (1.57–3.70) | |
| Cohort | 5 | 2.51 (1.34–4.70) | |
| NOS good | 12 | 1.93 (1.43–2.61) | |
| NOS fair/poor | 7 | 4.12 (3.09–5.51) | |
| Study (cohort) | Year | Selection representativeness of the sample | Selection of the non-intervention cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability based on design and analysis | Outcome assessment of outcome | Was follow up long enough for outcomes to occur | Adequacy of follow up of cohorts | Total score | Assessment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ravnskov | 1986 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 5 | Poor |
| Yaqoob et al. | 1993 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8 | Good |
| Radican et al. | 2006 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | Good |
| Jacob et al. | 2007 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 7 | Good |
| Jacob et al. | 2007 | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 7 | Good |
| Study (case-control) | Selection is the case definition adequate? | Representativeness of cases | Selection of controls | Definition of controls | Comparability based on design and analysis | Outcome assessment of exposure | Same method of ascertainment for cases and controls | Non-response rate | Total score | Assessment | |
| Zimmerman | 1975 | 1 | 1 | 0 | 1 | 2 | 0 | 1 | 0 | 6 | Poor |
| Ravnskov et al. | 1979 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 7 | Good |
| Finn et al. | 1980 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 4 | Poor |
| van der Laan | 1980 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 0 | 5 | Poor |
| Harrison et al. | 1986 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | Poor |
| Harrington et al. | 1989 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 | Good |
| Steenland et al. | 1990 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 8 | Good |
| Yaqoob et al. | 1992 | 1 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 6 | Fair |
| Porro et al. | 1992 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 0 | 7 | Good |
| Yaqoob et al. | 1994 | 1 | 0 | 0 | 1 | 2 | 1 | 1 | 0 | 6 | Fair |
| Stengel et al. | 1995 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | Poor |
| Nuyts et al. | 1995 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 7 | Good |
| Asal et al. | 1996 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 7 | Good |
| Fored et al. | 2004 | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 0 | 7 | Good |
| Outcome | Quality assessment | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Required domains | Additional domains | Grade | |||||||
| Study limitation | Consistency | Directness of evidence | Precision | Reporting bias | Dose-response association | Plausible confounding that would decrease observed effect | Strength of association (magnitude of effect) | ||
| CKD | Lowa | Inconsistentb | Direct | Precise | detectedc | None | Absent | Larged | ⨁⨁Low |
| Studies | Definition of high vs. low exposure | |
|---|---|---|
| Ravnskov | Intensity factor | |
| 2 = occupational house painting indoor, industrial spray-painting without protection devices, carpet and floor laying, production of paint and glue, polyester plastic application | ||
| 1 = non-occupational house painting indoor, spray-painting with protection devices, industrial degreasing of metal, printing work, occupational gluing, anesthesiologic work, dry cleaning | ||
| 0.5 = outdoor painting, motor repairing, handling of petrol, hobby gluing, drawing with filter-tipped pens | ||
| Intensity: Hour of exposure × Year of exposure × Intensity factor | ||
| 50 ≤ high exposed | ||
| 10 ≤ and < 50 exposed | ||
| < 10 non-exposed | ||
| Harrington et al. | Exposure indices (EIs) were computed for each relevant solvent type up to the time of diagnosis or retirement, whichever was the earlier. EI were then calculated by multiplying the score by the total duration of exposure, adjusted so that 1-year full time heavy exposure corresponded to an exposure index of 100. An overall EI for “total solvent” exposures was obtained simply by summing the EIs for individual solvents. | |
| 100 ≤ EI: Exposed | ||
| 1 ≤ EI < 100: Intermediate | ||
| EI < 1: Non-exposed | ||
| Porro et al. | Intensity factor | |
| 2 = production of paint and glue, occupational painting indoors, spray painting without protection devices, polyester resin application with heavy contact with styrene, tank cleaning without protection devices, carpet cleaning | ||
| 1 = non-occupational painting indoors, spray painting with protection devices, industrial degreasing of metals, printing work(rotogravure), occupational gluing(including vamps gluing), dry cleaning, anesthetic work, occupational use of hair sprays, use of pesticides, polyester resin application with low exposure to styrene | ||
| 0.5 = outdoor painting, motor repairing, hobby gluing, drawing with felt tipped pens, exposure to exhaust fumes outdoors, handling of petrol fuels, degreasing of metals(excluding industrial degreasing) | ||
| Intensity: Hour of exposure × Year of exposure × Intensity factor | ||
| High exposure = Above median | ||
| Lower exposure = Below median | ||
| No exposure = Reference | ||
| (median: occupational 80.12, non-occupational 2.76) | ||
| Stengel et al. | Two industrial hygiene experts assessed exposure to 30 different types of solvents as well as the level (low, medium, high) and the frequency of exposure (i.e., once a week, subdivided as < 2 h/wk, 2–20 h/wk, and > 20 h/wk) (The examples of method to calculate exposure level are not provided). | |
| No exposure: No exposure | ||
| Low exposure: Defined as < 2 h/wk whatever the level or low level whatever the frequency (< 2 h/wk or low level in the tables) | ||
| Fored et al. | The exposure was classified on a 5-level scale in terms of approximated additive HE. Exposure to an HE of 1.0 for a single solvent corresponds to an average exposure level during an 8-hour working day equal to the OEL prescribed by the Swedish Work Environment Authority in 1996 ( | |
| Cumulative lifetime exposure for organic solvents was calculated as the product of the intensity (HE), exposure frequency (days per month), and the duration (years) of the exposure, summed over all work periods in the subject’s occupational history. | ||
| Lifetime cumulative dose | ||
| First-Second quartile: Low exposure | ||
| Third-Fourth quartile: High exposure | ||
| Jacob et al. | Job periods were reviewed, and solvent exposure level (low, medium, high) and frequency (occasional [i.e., less than once a week] or regular [i.e., once a week], subdivided as < 2, 2 to 20, and > 20 h/wk) was determined by 2 industrial hygienists. (The examples of method to calculate exposure level are not provided) | |
| No exposure = No exposure | ||
| Low exposure = Low intensity or frequency < 2 h/wk. | ||
| High exposure = High intensity and frequency ≥ 2 h/wk. | ||
CKD: chronic kidney disease; GFR: glomerular filtration rate; ACR: albumin-to-creatinine ratio; RBD: red blood cell; WBC: white blood cell.
aACR conversion factor 1.0 mg/g = 0.113 mg/mmol.
ESRD: end-stage renal disease; GN: glomerulonephritis; ICD: International Statistical Classification of Diseases; GFR: glomerular filtration rate.
aAll papers with exposure defined as hydrocarbons included in this meta-analysis investigated hydrocarbons detected in petroleum-related products or paints, adhesives, degreasing agents, and diluents known to be occupationally exposed solvents.
bAmong several exposure factors, only hydrocarbon results were extracted and used for analysis.
OR: odds ratio; CI: confidence interval; GN: glomerulonephritis; NOS: Newcastle Ottawa Scale.
GRADE: Grading of Recommendations Assessment, Development and Evaluation; CKD: chronic kidney disease.
aAll included studies are observational, and the risk of bias in 5 included studies was evaluated as ‘Poor.’
bConsiderable heterogeneity (I2 = 87%).
cFunnel plot asymmetry, Egger’s regression test
dOverall pooled risk = 2.44.
HE: hygienic effect; OEL: occupational exposure limit.
 KSOEM
KSOEM

 Cite
Cite

