Articles
- Page Path
- HOME > Ann Occup Environ Med > Volume 33; 2021 > Article
- Review Tuberculosis infection status and risk factors among health workers: an updated systematic review
-
Sanga Lee1
 , Wanhyung Lee2
, Wanhyung Lee2 , Seong-Kyu Kang2
, Seong-Kyu Kang2
-
Annals of Occupational and Environmental Medicine 2021;33:e17.
DOI: https://doi.org/10.35371/aoem.2021.33.e17
Published online: May 28, 2021
1Gachon University College of Medicine, Incheon, Korea.
2Department of Occupational and Environmental Medicine, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea.
- Correspondence: Seong-Kyu Kang. Department of Occupational and Environmental Medicine, Gil Medical Center, Gachon University College of Medicine, 21 Namdong-daero 774beon-gil, Namdong-gu, Incheon 21565, Korea. sk.kang@gachon.ac.kr
Copyright © 2021 Korean Society of Occupational & Environmental Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Tuberculosis (TB) infection is a common occupational risk for health workers (HWs) and poses a threat to the patients under their care and to other HWs. Hence, the development of a prevention strategy is crucial. We conducted a study to understand the status and risk factors of TB infection among HWs. The existing literature was searched for all published reports from 1 August 2010 to 31 December 2018, related to TB among HWs according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The inclusion criteria were: (1) study participants working in a health care facility; (2) TB cases diagnosed by medical professionals; (3) original research articles; and (4) English reports in a peer-reviewed journal. We finally included 61 studies from 642 articles searched initially. The TB infection rate in HWs was higher than that of the general population. Based on 39 studies, the prevalence of TB in HWs (tuberculin skin test positive) was 29.94%. In contrast, the global burden of latent TB infection was 23.0% (95% uncertainty interval: 20.4%–26.4%) in 2014. The risk factors of TB among HWs were aging, long duration of employment, nursing professionals, lack of Bacillus Calmette-Guerin vaccination, and low body mass index. HWs have an increased risk for TB infection, which can cause secondary infections in patients or other HWs. An effective prevention strategy must be developed to enable early diagnosis and prompt treatment.
BACKGROUND
METHODS
RESULTS AND DISCUSSION
Summary of published studies meeting inclusion criteria for the TB among HWs
| Number | Author | Publication year | Country | Study design | Participants | Tuberculosis assessment | Prevalence or incidence |
|---|---|---|---|---|---|---|---|
| 1 | Chen et al. [16] | 2019 | China | Cross-sectional study | 487 | Questionnaire and IGRA for TB | IGRA positive: 33.9% |
| 2 | Nichimura et al. [51] | 2018 | Japan | Prospective cohort study | 328 | IGRA for TB | HW: 0.149/100 person-years |
| Nursing student: 0.0825/100 person-years | |||||||
| 3 | Bukhary et al. [52] | 2018 | Saudi Arabia | Cross-sectional study | 520 | IGRA for TB | QFT-GIT positive: 56/520 (10.8%) |
| 4 | Henderson et al. [7] | 2017 | UK | Cross-sectional study | 587 | IGRA for TB | IGRA positive: 27% |
| 5 | Shrestha et al. [22] | 2017 | Nepal | Cross-sectional study | 560 | Self-administered questionnaire and CXR | Direct contact with patients: 76.8% |
| Cough > 2 weeks: 10.4% | |||||||
| Chest radiography: 20.0% | |||||||
| 6 | Lacerda et al. [25] | 2017 | Brazil | Cross-sectional study | 708 | Questionnaire and IGRA | LTBI prevalence: 27% (n = 196; 95% CI: 24%–31%) |
| 7 | Lacerda et al. [25] | 2017 | Brazil | Cross-sectional study | 218 | TST | TST positive: 39.4% (95% CI: 32.9–45.9) and 54.1% (95% CI: 47.4–60.7) |
| 8 | Yoon et al. [32] | 2017 | Korea | Cross-sectional study | 902 | TST, IGRA | TB patient care ≥ 1 year or more: 19.5% (176/902) |
| TST positive: 26.9% (243/902) | |||||||
| LTBI: 5.8% (52/902) | |||||||
| 9 | Janagond et al. [24] | 2017 | India | Prospective cohort study | 206 | Questionnaires, TST | TST positive: 36.8% (76/206) |
| 10 | Napoli et al. [5] | 2017 | Italy | Cross-sectional study | 2,290 | QFT, TST | TST positive: 141 (6.1%) |
| QFT positive: 16.4% | |||||||
| 11 | Gehanno et al. [40] | 2017 | France | Retrospective study | 233,389 | Questionnaires | Nurse, health care assistants: 5.7/100,000 |
| Administrative staff: 1.27/100,000 | |||||||
| 12 | Pan et al. [13] | 2015 | Taiwan | Matched cohort study | 44 | AFB staining | Smear positive: 26.8% (11/41) |
| Culture proven: 70.7% (29/41) | |||||||
| Pathologically diagnosed: 9.8% (4/41) | |||||||
| Clinically diagnosed: 24.4% (10/41) | |||||||
| 13 | He et al. [17] | 2015 | China | Longitudinal study | 880 | TST, QFT | TST positive: 11.4% |
| QFT-GIT: 19.1% (OR: 142.62, 95% CI: 1.39–4.97) | |||||||
| BCG scar: OR: 0.53, 95% CI: 0.28–1.00 | |||||||
| 14 | Adams et al. [33] | 2015 | South Africa | Prospective cohort study | 764 | TST, QFT, CXR | TST positive: 38% |
| QFT-GIT positive: 13%–22% | |||||||
| 15 | Hung et al. [14] | 2015 | Taiwan | Prospective cross-sectional study | 193 | QFT-G, TST | TST positive: 88.8% |
| QFT-GIT positive: 14.5% | |||||||
| Multivariable logistic regression: only the QFT-G test was associated with age (35 years or greater) (adjusted OR: 2.53, p = 0.03) | |||||||
| 16 | Chen et al. [31] | 2014 | China | Retrospective study | 996 | Sputum smear | TB: females > males (58.0% > 42.0%) |
| TB positive: males > females (88.5% > 83.4%, p = 0.031) | |||||||
| 17 | Garcell et al. [34] | 2014 | Qatar | Cross-sectional study | 202 | TST, QFT-G | TST positive: 6.9% |
| QFT-G positive: 3.0% | |||||||
| TB positive is older than negative (44.5 vs. 38.9 years) | |||||||
| More experience as HWs (21.7 vs. 16.8 years) | |||||||
| Direct contact with Tb patients (83.3% vs. 25%) | |||||||
| 18 | Szep et al. [27] | 2014 | USA | Cross-sectional study | 95 | TST | TST positive: 4.2% or 6.87 per 1,000 person weeks (95% CI: 1.87–17.60). |
| 19 | Muzzi et al. [6] | 2014 | Italy | Cross-sectional study | 388 | TST | TST at T1 was positive: 11 (4.3%). ARTI was 1.6 (95% CI: 0.9–2.9) per 100 person-years |
| TST at T2 was positive: 9 (3.7%) | |||||||
| HWs PEARTI was 26 (95% CI 13.6–50) per 100 person-years | |||||||
| 20 | Wei et al. [18] | 2013 | China | Observational study | 210 | IGRA, TST | QFT-GIT positive: 161 (76.7%) |
| QFT-GIT negative: 10 (19.01%) | |||||||
| Indeterminate results: 9 (4.3%) | |||||||
| (κ = 0.456, p < 0.001) | |||||||
| 21 | Gran et al. [8] | 2013 | Norway | Cross-sectional study | 387 | QFT, TST | QFT-GIT positive: 3.4% |
| TST (≥ 6 mm): 214 (55.3%) | |||||||
| TST (≥ 15 mm): 53 (13.7%) | |||||||
| QFT, TST positive: 10 (4.7%) | |||||||
| 22 | Whitaker et al. [9] | 2013 | Georgia | Prospective longitudinal study | 319 | TST, QFT-GIT | HWs from TB unit had higher prevalence of positive QFT-GIT and TST than those from non-TB unit: 107/194 (55%) vs. 30/125 (31%) QFT-GIT positive (p < 0.0001) and 128/189 (69%) vs. 64/119 (54%) TST positive (p = 0.01) |
| 23 | Kiertiburanakul et al. [15] | 2012 | Thailand | Prospective study | 1,438 | TST | TST positive: 66.3% |
| TST conversion: 4.8 per 100 HCP-year | |||||||
| 9 (0.6%) HCP were diagnosed with active TB | |||||||
| 24 | Larcher et al. [53] | 2012 | Italy | Cross-sectional study | 621 | TST, QFT | TST positive: 29.1% |
| QFT positive: 18.5% | |||||||
| 25 | Zwerling et al. [29] | 2012 | Canada | Prospective longitudinal study | 388 | TST, QFT | TST positive: 5.7% (22/388, 95% CI: 3.6%–8.5%) |
| QFT positive: 6.2% (24/388, 95% CI: 4%–9.1%) | |||||||
| 26 | Borroto et al. [30] | 2011 | Cuba | Cross-sectional study | 350 | TST | LTBI prevalence: 15.4%: it was highest in professionals (20.6%); 60.3% were non-reactors, and at the second test a year later 1.4% were converters |
| 27 | Moon et al. [21] | 2011 | Korea | Cross-sectional study | 173 | TST, QFT | QFT-GIT positive: 21.4% |
| TST positive: 33.3% | |||||||
| κ = 0.234 | |||||||
| 28 | Sherman et al. [10] | 2011 | Germany | Retrospective cohort study | 450 | TST | TST conversion: 93 |
| 29 | Kehinde et al. [54] | 2011 | Nigeria | Descriptive study | 271 | Pre-tested questionnaire | AFB stain positive: 9 (3.3%) |
| Culture positive: 6 (2.2%) | |||||||
| The culture contamination: 1.8 per cent | |||||||
| 30 | Costa et al. [12] | 2011 | Portugal | Cross-sectional study | 376 | QFT, TST | TST positive: 61 |
| 31 | Rafiza et al. [23] | 2011 | Malaysia | Cross-sectional study | 954 | QFT, TST | The overall prevalence of latent tuberculosis infection among Health workers was 10.6% (CI: 8.6%–12.6%) |
| 32 | Park et al.[55] | 2010 | Korea | Prospective study | 322 | QFT, TST | Both positive: 25 subjects (8%) |
| Follow-up after 1 year | |||||||
| QFT-GIT positive: between 3.3% and 5.7% | |||||||
| 33 | Cadmus et al. [56] | 2010 | Nigeria | Retrospective study | 101 | AFB | AFB positive: 10 (13%) |
| 34 | Escombe et al. [26] | 2010 | Peru | Cross-sectional study | 845 | QFT | QFT-GIT positive: 39 (56%) |
| 35 | Lambert et al. [28] | 2012 | USA | Cross-sectional study | 200,744 | TST | TST positive: 6,049 (3%) |
| 36 | Schablon et al. [11] | 2010 | Germany | Cross-sectional study | 2,028 | IGRA | QFT-GIT positive: 198 (9.9%) |
| TST positive: 480 (24.0%) | |||||||
| 37 | Jo et al. [20] | 2013 | Korea | Cross-sectional study | 493 | TST, QFT | Doctors (n = 99): TST positive: 63 (41.4%)/QFT-GIT positive: 36 (23.7%) |
| Nurse (n = 168): TST positive: 119 (34.9%)/QFT-positive: 49 (14.4%) |
Summary of published studies meeting inclusion criteria for risk factors of TB among HWs
| Number | Author | Year | Country | Study design | Participants | Tuberculosis assessment | Estimate of risk |
|---|---|---|---|---|---|---|---|
| 1 | Wang et al. [35] | 2018 | China | Cross-sectional study | 212 | Positive sputum acid-fast stains | TB: 760/100,000, RF: 51 years and above (aOR: 6.17, 95% CI: 1.35–28.28), being a nurse (aOR: 3.09, 95% CI: 1.15–8.32) |
| 2 | Kim et al. [19] | 2017 | Korea | Prospective cohort study | 872 | TST, CXR | Age over 30 years: (p = 0.02), LTBI point prevalence: 6.6%, LTBI incidence: 2.4 per 100 HWs |
| 3 | Davidson et al. [57] | 2017 | UK | Retrospective cohort study | 2,320 | TB surveillance, genotyping data | HWs: 23.4 (95% CI: 22.5–24.4), non-HWs: 16.2 (95% CI: 16.0–16.3) |
| 4 | Belo et al. [38] | 2017 | Mozambique | Cross-sectional study | 316 | Symptom screening questionnaire | LTBI: 34.4%, working > 8 years: 39.3%, no BCG vaccine: 39.6%, immunocompromised: 78.1% |
| 5 | Du et al. [48] | 2017 | China | Cross-sectional study | 186 | Questionnaires | Medical professionals (PR = 2.40), laboratory technicians (PR = 2.17), other hospital staff (PR = 1.04) |
| 6 | Bonini et al. [39] | 2017 | Italy | Cross-sectional study | 580 | Questionnaires, TST | Previous BCG vaccination: OR: 344, CI: 43.72–2,718.41, p < 0.001, origin in high-risk countries: OR: 401.68, CI: 50.60–3,188.69, p < 0.001 |
| 7 | Weng et al. [58] | 2016 | Swaziland | Cross-sectional study | 186 | Questionnaires | Nurses (OR: 39.87, 95% CI: 2.721–584.3), other HWs (OR: 99.34, 95% CI: 7.469–1,321) |
| 8 | Nonghanphithak et al. [36] | 2016 | Thailand | Cross-sectional study | 112 | QFT, questionnaires | Age ≥ 30 years (OR: 18.88, 95% CI: 1.52–234.36), nurse (OR: 2.78, 95% CI: 1.19–6.49), job for ≥ 10 years (OR: 8.78, 95% CI: 1.26–61.29) |
| 9 | Tudor et al. [43] | 2016 | South Africa | Case-control study | 307 | Questionnaires | HWs living with HIV (OR: 6.35, 95% CI: 3.54–11.37) spent time working in areas with patients (OR: 2.24; 95% CI: 1.40–3.59) |
| 10 | Ito et al. [41] | 2016 | Japan | Retrospective study | 875 | IGRA, CXR | Multivariate analysis (OR: 8.2, 95% CI: 1.3–78.3, p = 0.03), longer duration of contact (> 7 days, 12/12 [100%], vs. ≤ 7 days, 18/43 [41.9%]; p = 0.0002), fewer symptoms (> 7 days, 5/12 [41.7%] vs. ≤ 7 days, 35/43 [81.4%]; p = 0.01). |
| 11 | Tsang et al. [59] | 2015 | Hong Kong | Prospective cohort study | 279 | IGRA, QFT | QFT-GIT positive: (exposed: 19.5%, non-exposed: 20.8%, RR = 0.96, 95% CI: 0.74–1.25, p > 0.05) |
| 12 | Agaya et al. [60] | 2015 | Kenya | Cross-sectional survey | 1,416 | Standardized questionnaire | LTBI prevalence: (p = 0.72), work year: p < 0.01 |
| 13 | McCarthy et al. [61] | 2015 | South Africa | Cross-sectional study | 199 | IGRA, TST | Incident LTBI (IGRA): 25/97 (26%; incident rate 29 cases/100 person-years, 95% CI: 20–44), TST: 25/93 (27%; incident rate 29 cases/100 person-years, 95% CI: 19–42) |
| 14 | Rutanga et al. [62] | 2015 | Rwanda | Cross-sectional study | 1,131 | TST | LTBI prevalence: (62.1%), TST positive odds TST: 2.71 times greater (95% CI: 2.01–3.67), work year odds: increasing 4% (aOR: 1.04, 95% CI: 1.02%–1.05%) per year |
| 15 | Chu et al. [63] | 2014 | Taiwan | Population-based cohort study | 11,811 | Chart review | TB incidence: HWs vs matched subjects (61.08 vs. 37.81 per 100,000 person-years) |
| Risk of TB: HWs (aHR: 1.62, 95% CI: 1.08–2.43) | |||||||
| 16 | Tudor et al. [43] | 2014 | South Africa | Retrospective cohort study | 1,313 | Chart review | HWs living with HIV had a greater incidence of TB (IRR: 3.2, 95% CI: 1.54–6.66) than HIV-negative HWs |
| 17 | Zhou et al. [64] | 2014 | China | Cross-sectional study | 712 | TST | TB hospital: 58.0% (n = 127), non-TB hospital: 33.9% (n = 105) (OR: 2.40, 95% CI:1.59–3.62), (6–10 years vs. ≤ 5 years [OR: 1.89, 95% CI: 1.10–3.25] and > 10 vs. ≤ 5 [OR:1.80; 95% CI: 1.20–2.68]) |
| 18 | Claassens et al. [65] | 2013 | South Africa | Cross-sectional study | 133 | Sputum smear | The infection control audit score: OR: 1.04, 95% CI: 1.01–1.08, p = 0.02, the number of staff: OR: 3.78, 95% CI: 1.77–8.08, the number of staff remained: OR: 3.33, 95% CI: 1.37–8.08 |
| 19 | Durando et al. [66] | 2013 | Italy | Cross-sectional study | 881 | TST | Born in high TB incidence areas (≥ 20 cases per 100,000 population) |
| 20 | Kim et al. [37] | 2013 | Korea | Cross-sectional study | 2,132 | TST | TST positive: 778 (36.5%), being older (OR: 1.10, 95% CI: 1.06–1.13, p < 0.001), male (OR: 1.78, 95% CI: 1.21–2.62, p = 0.003), re-joining the hospital workforce (OR: 1.58, 95% CI: 1.04–2.40, p = 0.032) |
| 21 | Casas et al. [67] | 2013 | Spain | Cohort analysis | 614 | TST | High risk worker hazard ratio: 1.55 (95% CI: 1.05–2.27) gender, age and professional status |
| 22 | Mathew et al. [42] | 2013 | South India | Case-control study | 101 | TST | BMI < 19 kg/m2 (OR: 2.96, 95% CI: 1.49–5.87), contact with patients (OR: 2.83, 95% CI: 1.47–5.45), being employed in medical wards (OR: 12.37, 95% CI: 1.38–110.17), microbiology laboratories (OR: 5.65, 95% CI: 1.74–18.36) |
| 23 | He et al. [68] | 2012 | China | Cross-sectional study | 999 | TST, QFT-GIT | QFT-GIT-positive: 683 (68%) associated with greater age, longer HW career, TB disease in a co-worker and greater daily patient exposure using multivariable analysis |
Abbreviations
AFB
aOR
ARTI
BCG
BMI
CI
CXR
GIT
HIV
HR
HW
IGRA
IRR
LTBI
OR
PR
QFT
QTF-PEARTI
RF
RR
TB
TST
WHO
-
Competing interests: The authors declare that they have no competing interest.
-
Author Contributions:
NOTES
- 1. Zwerling A, van den Hof S, Scholten J, Cobelens F, Menzies D, Pai M. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax 2012;67(1):62–70. 21228420.ArticlePubMed
- 2. Dharmadhikari AS, Mphahlele M, Venter K, Stoltz A, Mathebula R, Masotla T, et al. Rapid impact of effective treatment on transmission of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2014;18(9):1019–1025. 25189547.ArticlePubMedPMC
- 3. von Delft A, Dramowski A, Khosa C, Kotze K, Lederer P, Mosidi T, et al. Why healthcare workers are sick of TB. Int J Infect Dis 2015;32:147–151. 25809771.ArticlePubMed
- 4. Baussano I, Nunn P, Williams B, Pivetta E, Bugiani M, Scano F. Tuberculosis among health care workers. Emerg Infect Dis 2011;17(3):488–494. 21392441.ArticlePubMedPMC
- 5. Napoli C, Ferretti F, Di Ninno F, Orioli R, Marani A, Sarlo MG, et al. Screening for tuberculosis in health care workers: experience in an Italian teaching hospital. BioMed Res Int 2017;2017:7538037. 28337457.ArticlePubMedPMCPDF
- 6. Muzzi A, Seminari E, Feletti T, Scudeller L, Marone P, Tinelli C, et al. Post-exposure rate of tuberculosis infection among health care workers measured with tuberculin skin test conversion after unprotected exposure to patients with pulmonary tuberculosis: 6-year experience in an Italian teaching hospital. BMC Infect Dis 2014;14(1):324. 24919953.ArticlePubMedPMCPDF
- 7. Henderson M, Howard SJ. Screening for latent tuberculosis in UK health care workers. Occup Med (Lond) 2017;67(8):641–643. 29016903.ArticlePubMed
- 8. Gran G, Aßmus J, Dyrhol-Riise AM. Screening for latent tuberculosis in Norwegian health care workers: high frequency of discordant tuberculin skin test positive and interferon-gamma release assay negative results. BMC Public Health 2013;13(1):353. 23590619.ArticlePubMedPMCPDF
- 9. Whitaker JA, Mirtskhulava V, Kipiani M, Harris DA, Tabagari N, Kempker RR, et al. Prevalence and incidence of latent tuberculosis infection in georgian healthcare workers. PLoS One 2013;8(3):e58202. 23536789.ArticlePubMedPMC
- 10. Sherman HA, Karakis I, Heimer D, Arzt M, Goldstein W, Bouhnik L, et al. Housekeeping health care workers have the highest risk for tuberculin skin test conversion. Int J Tuberc Lung Dis 2011;15(8):1050–1055. 21740667.ArticlePubMed
- 11. Schablon A, Harling M, Diel R, Nienhaus A. Risk of latent TB infection in individuals employed in the healthcare sector in Germany: a multicentre prevalence study. BMC Infect Dis 2010;10(1):107. 20429957.ArticlePubMedPMCPDF
- 12. Torres Costa J, Silva R, Sá R, Cardoso MJ, Nienhaus A. Serial testing with the interferon-γ release assay in Portuguese healthcare workers. Int Arch Occup Environ Health 2011;84(4):461–469. 20721576.ArticlePubMedPMC
- 13. Pan SC, Chen YC, Wang JY, Sheng WH, Lin HH, Fang CT, et al. Tuberculosis in healthcare workers: a matched cohort study in Taiwan. PLoS One 2015;10(12):e0145047. 26679188.ArticlePubMedPMC
- 14. Hung WT, Lee SS, Sy CL, Wu KS, Chen JK, Tsai HC, et al. Prevalence of latent tuberculosis infection in BCG-vaccinated healthcare workers by using an interferon-gamma release assay and the tuberculin skin test in an intermediate tuberculosis burden country. J Microbiol Immunol Infect 2015;48(2):147–152. 24071516.ArticlePubMed
- 15. Kiertiburanakul S, Suebsing S, Kehachindawat P, Apivanich S, Somsakul S, Sathapatayavongs B, et al. Five-year prospective study of tuberculin skin testing among new healthcare personnel at a university hospital in Thailand. J Hosp Infect 2012;80(2):173–175. 22192172.ArticlePubMed
- 16. Chen B, Gu H, Wang X, Wang F, Peng Y, Ge E, et al. Prevalence and determinants of latent tuberculosis infection among frontline tuberculosis healthcare workers in southeastern China: a multilevel analysis by individuals and health facilities. Int J Infect Dis 2019;79:26–33. 30471404.ArticlePubMed
- 17. He G, Li Y, Zhao F, Wang L, Cheng S, Guo H, et al. The prevalence and incidence of latent tuberculosis infection and its associated factors among village doctors in China. PLoS One 2015;10(5):e0124097. 25996960.ArticlePubMedPMC
- 18. Wei Z, Yang M, Quan B, Wang Y, Wu Y, Ji B. Prevalence of latent tuberculosis infection among healthcare workers in China as detected by two interferon-gamma release assays. J Hosp Infect 2013;84(4):323–325. 23806839.ArticlePubMed
- 19. Kim YJ, Chi YH, Lee JY, Lee HJ, Kang JY, Kim YR, et al. In-hospital contact investigation among health care workers after exposure to pulmonary tuberculosis in an intermediate tuberculosis prevalence area: a prospective study. Arch Environ Occup Health 2017;72(5):272–278. 27471918.ArticlePubMed
- 20. Jo KW, Hong Y, Park JS, Bae IG, Eom JS, Lee SR, et al. Prevalence of latent tuberculosis infection among health care workers in South Korea: a multicenter study. Tuberc Respir Dis 2013;75(1):18–24.ArticlePubMedPMC
- 21. Moon HW, Kim H, Hur M, Yun YM, Lee A. Latent tuberculosis infection screening for laboratory personnel using interferon-γ release assay and tuberculin skin test in Korea: an intermediate incidence setting. J Clin Lab Anal 2011;25(6):382–388. 22086790.ArticlePubMedPMC
- 22. Shrestha P, Shakya M, Caws M, Shrestha S, Karki B, Shrestha S, et al. Tuberculosis in staff and students of Patan hospital. J Nepal Health Res Counc 2018;15(3):268–274. 29353901.ArticlePubMed
- 23. Rafiza S, Rampal KG, Tahir A. Prevalence and risk factors of latent tuberculosis infection among health care workers in Malaysia. BMC Infect Dis 2011;11(1):19. 21244645.ArticlePubMedPMCPDF
- 24. Janagond AB, Ganesan V, Vijay Kumar GS, Ramesh A, Anand P, Mariappan M. Screening of health-care workers for latent tuberculosis infection in a Tertiary Care Hospital. Int J Mycobacteriol 2017;6(3):253–257. 28776523.ArticlePubMed
- 25. Lacerda TC, Souza FM, Prado TN, Locatelli RL, Fregona G, Lima RC, et al. Tuberculosis infection among primary health care workers. J Bras Pneumol 2017;43(6):416–423. 29340489.ArticlePubMedPMC
- 26. Escombe AR, Huaroto L, Ticona E, Burgos M, Sanchez I, Carrasco L, et al. Tuberculosis transmission risk and infection control in a hospital emergency department in Lima, Peru. Int J Tuberc Lung Dis 2010;14(9):1120–1126. 20819256.PubMed
- 27. Szep Z, Kim R, Ratcliffe SJ, Gluckman S. Tuberculin skin test conversion rate among short-term health care workers returning from Gaborone, Botswana. Travel Med Infect Dis 2014;12(4):396–400. 23932600.ArticlePubMed
- 28. Lambert LA, Pratt RH, Armstrong LR, Haddad MB. Tuberculosis among healthcare workers, United States, 1995-2007. Infect Control Hosp Epidemiol 2012;33(11):1126–1132. 23041811.ArticlePubMedPMC
- 29. Zwerling A, Cojocariu M, McIntosh F, Pietrangelo F, Behr MA, Schwartzman K, et al. TB screening in Canadian health care workers using interferon-gamma release assays. PLoS One 2012;7(8):e43014. 22916197.ArticlePubMedPMC
- 30. Borroto S, Gámez D, Díaz D, Martínez Y, Ferrer AI, Velásquez Y, et al. Latent tuberculosis infection among health care workers at a general hospital in Santiago de Cuba. Int J Tuberc Lung Dis 2011;15(11):1510–1514. 22008765.ArticlePubMed
- 31. Chen B, Wang X, Zhong J, Chen S, Wu B, Yeh HC, et al. Tuberculosis among healthcare workers in southeastern China: a retrospective study of 7-year surveillance data. Int J Environ Res Public Health 2014;11(11):12042–12052. 25419877.ArticlePubMedPMC
- 32. Yoon CG, Oh SY, Lee JB, Kim MH, Seo Y, Yang J, et al. Occupational risk of latent tuberculosis infection in health workers of 14 military hospitals. J Korean Med Sci 2017;32(8):1251–1257. 28665059.ArticlePubMedPMCPDF
- 33. Adams S, Ehrlich R, Baatjies R, van Zyl-Smit RN, Said-Hartley Q, Dawson R, et al. Incidence of occupational latent tuberculosis infection in South African healthcare workers. Eur Respir J 2015;45(5):1364–1373. 25700382.ArticlePubMedPMC
- 34. Guanche Garcell H, Crespo Ramirez E, Kindelan Contreras A, Gutierrez Garcia F. Latent tuberculosis infection in healthcare workers at a community hospital in Qatar. J Infect Public Health 2014;7(4):356–359. 24702746.ArticlePubMed
- 35. Wang XN, He TL, Geng MJ, Song YD, Wang JC, Liu M, et al. Prevalence of and risk factors for tuberculosis among healthcare workers in Chinese tuberculosis facilities. Infect Dis Poverty 2018;7(1):26. 29592797.ArticlePubMedPMCPDF
- 36. Nonghanphithak D, Reechaipichitkul W, Chaiyasung T, Faksri K. Risk factors for latent tuberculosis infection among health-care workers in northeastern thailand. Southeast Asian J Trop Med Public Health 2016;47(6):1198–1208. 29634186.PubMed
- 37. Kim SY, Park MS, Kim YS, Kim SK, Chang J, Yong D, et al. Tuberculin skin test and boosted reactions among newly employed healthcare workers: an observational study. PLoS One 2013;8(5):e64563. 23717631.ArticlePubMedPMC
- 38. Belo C, Naidoo S. Prevalence and risk factors for latent tuberculosis infection among healthcare workers in Nampula Central Hospital, Mozambique. BMC Infect Dis 2017;17(1):408. 28595594.ArticlePubMedPMCPDF
- 39. Bonini S, Riccelli MG, Goldoni M, Selis L, Corradi M. Risk factors for latent tuberculosis infection (LTBI) in health profession's students of the University of Parma. Acta Biomed 2017;88(1S):54–60.
- 40. Gehanno JF, Abiteboul D, Rollin L. Incidence of tuberculosis among nurses and healthcare assistants in France. Occup Med (Lond) 2017;67(1):58–60. 27694378.ArticlePubMed
- 41. Ito Y, Nagao M, Iinuma Y, Matsumura Y, Yamamoto M, Takakura S, et al. Risk factors for nosocomial tuberculosis transmission among health care workers. Am J Infect Control 2016;44(5):596–598. 26777287.ArticlePubMed
- 42. Mathew A, David T, Thomas K, Kuruvilla PJ, Balaji V, Jesudason MV, et al. Risk factors for tuberculosis among health care workers in South India: a nested case-control study. J Clin Epidemiol 2013;66(1):67–74. 22521578.ArticlePubMed
- 43. Tudor C, Van der Walt ML, Margot B, Dorman SE, Pan WK, Yenokyan G, et al. Occupational risk factors for tuberculosis among healthcare workers in KwaZulu-Natal, South Africa. Clin Infect Dis 2016;62(Suppl 3):S255–S261. 27118855.ArticlePubMedPMC
- 44. Al Hajoj S, Varghese B, Datijan A, Shoukri M, Alzahrani A, Alkhenizan A, et al. Interferon gamma release assay versus tuberculin skin testing among healthcare workers of highly diverse origin in a moderate tuberculosis burden country. PLoS One 2016;11(5):e0154803. 27148876.ArticlePubMedPMC
- 45. Christopoulos AI, Diamantopoulos AA, Dimopoulos PA, Goumenos DS, Barbalias GA. Risk factors for tuberculosis in dialysis patients: a prospective multi-center clinical trial. BMC Nephrol 2009;10(1):36. 19895701.ArticlePubMedPMCPDF
- 46. Shetty N, Shemko M, Vaz M, D'Souza G. An epidemiological evaluation of risk factors for tuberculosis in South India: a matched case control study. Int J Tuberc Lung Dis 2006;10(1):80–86. 16466042.PubMed
- 47. World Health Organization. Guidelines on the Management of Latent Tuberculosis Infection. Geneva, Switzerland: WHO; 2015.
- 48. Cloete B, Yassi A, Ehrlich R. Repeat auditing of primary health-care facilities against standards for occupational health and infection control: a study of compliance and reliability. Saf Health Work 2020;11(1):10–18. 32206369.ArticlePubMedPMC
- 49. Liautaud A, Adu PA, Yassi A, Zungu M, Spiegel JM, Rawat A, et al. Strengthening human immunodeficiency virus and tuberculosis prevention capacity among South African healthcare workers: a mixed methods study of a collaborative occupational health program. Saf Health Work 2018;9(2):172–179. 29928531.ArticlePubMedPMC
- 50. World Health Organization. Global tuberculosis programme and global programme on vaccines. Statement on BCG revaccination for the prevention of tuberculosis. Wkly Epidemiol Rec 1995;70(32):229–231. 7669527.PubMed
- 51. Nishimura T, Ota M, Mori M, Fujiwara H, Takano Y, Kato S, et al. Risk of tuberculosis infection among health care workers and nursing students in Japan. J Infect Chemother 2018;24(11):921–924. 30181031.ArticlePubMed
- 52. Bukhary ZA, Amer SM, Emara MM, Abdalla ME, Ali SA. Screening of latent tuberculosis infection among health care workers working in Hajj pilgrimage area in Saudi Arabia, using interferon gamma release assay and tuberculin skin test. Ann Saudi Med 2018;38(2):90–96. 29620541.ArticlePubMedPMC
- 53. Larcher C, Frizzera E, Pretto P, Lang M, Sonnleitner N, Huemer HP. Immunosurveillance for Mycobacterium tuberculosis of health care personnel in a third level care hospital. Med Lav 2012;103(1):26–36. 22486073.PubMed
- 54. Kehinde AO, Baba A, Bakare RA, Ige OM, Gbadeyanka CF, Adebiyi OE. Pulmonary tuberculosis among health care workers at two designated DOTS Centers in urban city of Ibadan, Nigeria. Indian J Med Res 2011;133(6):613–617. 21727659.PubMedPMC
- 55. Park HY, Jeon K, Suh GY, Kwon OJ, Chung DR, Yoonchang SW, et al. Interferon-γ release assay for tuberculosis screening of healthcare workers at a Korean tertiary hospital. Scand J Infect Dis 2010;42(11-12):943–945. 20936910.ArticlePubMed
- 56. Cadmus SI, Okoje VN, Taiwo BO, van Soolingen D. Exposure of dentists to Mycobacterium tuberculosis, Ibadan, Nigeria. Emerg Infect Dis 2010;16(9):1479–1481. 20735939.ArticlePubMedPMC
- 57. Davidson JA, Lalor MK, Anderson LF, Tamne S, Abubakar I, Thomas HL. TB in healthcare workers in the UK: a cohort analysis 2009-2013. Thorax 2017;72(7):654–659. 26888779.ArticlePubMed
- 58. Weng YH, Bhembe PT, Chiou HY, Yang CY, Chiu YW. Perceived risk of tuberculosis infection among healthcare workers in Swaziland. BMC Infect Dis 2016;16(1):697. 27881088.ArticlePubMedPMCPDF
- 59. Tsang DN, Lai CK, Yam WC, Chan JW, Mok YW, Seto WH, et al. Use of interferon gamma release assay to assess latent tuberculosis infection among healthcare workers in Hong Kong. Hong Kong Med J 2015;21(Suppl 7):S22–S25. 26908269.PubMed
- 60. Agaya J, Nnadi CD, Odhiambo J, Obonyo C, Obiero V, Lipke V, et al. Tuberculosis and latent tuberculosis infection among healthcare workers in Kisumu, Kenya. Trop Med Int Health 2015;20(12):1797–1804. 26376085.PubMed
- 61. McCarthy KM, Scott LE, Gous N, Tellie M, Venter WD, Stevens WS, et al. High incidence of latent tuberculous infection among South African health workers: an urgent call for action. Int J Tuberc Lung Dis 2015;19(6):647–653. 25946353.ArticlePubMed
- 62. Rutanga C, Lowrance DW, Oeltmann JE, Mutembayire G, Willis M, Uwizeye CB, et al. Latent tuberculosis infection and associated factors among Health Care Workers in Kigali, Rwanda. PLoS One 2015;10(4):e0124485. 25919759.ArticlePubMedPMC
- 63. Chu H, Shih CJ, Lee YJ, Kuo SC, Hsu YT, Ou SM, et al. Risk of tuberculosis among healthcare workers in an intermediate-burden country: a nationwide population study. J Infect 2014;69(6):525–532. 25135230.ArticlePubMed
- 64. Zhou F, Zhang L, Gao L, Hao Y, Zhao X, Liu J, et al. Latent tuberculosis infection and occupational protection among health care workers in two types of public hospitals in China. PLoS One 2014;9(8):e104673. 25157814.ArticlePubMedPMC
- 65. Claassens MM, van Schalkwyk C, du Toit E, Roest E, Lombard CJ, Enarson DA, et al. Tuberculosis in healthcare workers and infection control measures at primary healthcare facilities in South Africa. PLoS One 2013;8(10):e76272. 24098461.ArticlePubMedPMC
- 66. Durando P, Sotgiu G, Spigno F, Piccinini M, Mazzarello G, Viscoli C, et al. Latent tuberculosis infection and associated risk factors among undergraduate healthcare students in Italy: a cross-sectional study. BMC Infect Dis 2013;13(1):443. 24059355.ArticlePubMedPMCPDF
- 67. Casas I, Esteve M, Guerola R, García-Olivé I, Roldán-Merino J, Martinez-Rivera C, et al. Incidence of tuberculosis infection among healthcare workers: risk factors and 20-year evolution. Respir Med 2013;107(4):601–607. 23312619.ArticlePubMed
- 68. He GX, Wang LX, Chai SJ, Klena JD, Cheng SM, Ren YL, et al. Risk factors associated with tuberculosis infection among health care workers in Inner Mongolia, China. Int J Tuberc Lung Dis 2012;16(11):1485–1491. 22964074.ArticlePubMed
REFERENCES
REFERENCES
Figure & Data
REFERENCES
Citations

- Prevalence of Mycobacterium tuberculosis and risk factors among internally and externally displaced populations in northwestern Ethiopia: The case of Dabat and Metema
Deresse Daka, Belay Tessema, Awelani Mutshembele, Amir Alelign, Wubet Birhan, Baye Gelaw
IJID Regions.2026; 18: 100836. CrossRef - Impact of WHO-recommended tuberculosis control measures on occupational tuberculosis risk among healthcare workers in a high-burden tertiary hospital in Brazil: a 24-year retrospective analysis
P. Bortolozzi-Mendes, M. Rennó de Campos, H. de Oliveira Couto, M.C. Vieira de Almeida, J.G.C. Gonçalves de Oliveira, F. Bellissimo-Rodrigues, C.H. Miranda, A. Pazin-Filho
Journal of Hospital Infection.2025; 157: 75. CrossRef - Increased autophagy activity regulated by LC3B gene promoter DNA methylation is associated with progression to active pulmonary tuberculosis disease
Yung-Che Chen, Ying-Tang Fang, Chao-Chien Wu, Tung-Ying Chao, Yi-Hsi Wang, Chia-Cheng Tseng, Sum-Yee Leung, Chiu-Ping Lee, Ting-Ya Wang, Po-Yuan Hsu, Jen-Chieh Chang, Meng-Chih Lin, Chang-Chun Hsiao
Respiratory Research.2025;[Epub] CrossRef - An interoperable web-based platform to support health surveillance against latent tuberculosis infection in health care workers and students: The evolution of CROSSWORD study protocol
Angela Rizzi, Eleonora Nucera, Walter Mazzucco, Pierpaolo Palumbo, Domenico Staiti, Umberto Moscato, Francesco Maria De Simone, Michela Sali, Luca Boldrini, Nikola Dino Capocchiano, Stefano Patarnello, Gabriele Rumi, Raffaella Chini, Valentina Carusi, Mic
PLOS ONE.2025; 20(3): e0319568. CrossRef - Prevalence and risk factors of latent tuberculosis infection in healthcare workers in Eastern China
Lili Zhen, Shichao Shangguan, Jing Zhou, Shulei Wang, Liyong Lu, Yuelei Wang, Jingyu Liu
BMC Public Health.2025;[Epub] CrossRef - Establishing consensus of perceived occupational risk of tuberculosis (POR-TB) among healthcare workers: a Delphi study in Indonesia
Agus Fitriangga, Wilson Wilson, Risa Febriana Musawaris, Desni Yuniarni, Fitri Sukmawati, Risnawati Risnawati, Patricia Ami, Rudi Anshari, Insanul Kamilah, Irma Prasetyowati, Nurmainah Nurmainah, Alex Alex
Discover Public Health.2025;[Epub] CrossRef - Prevalence, Risk Factors, and Result Features in the Detection of Latent Tuberculosis Infection in Thai Healthcare Workers Using QuantiFERON-TB Gold Plus
Wiphat Klayut, Sopa Srisungngam , Sirilada Suphankong, Pantip Sirichote, Benjawan Phetsuksiri, Supranee Bunchoo, Chiranan Jakreng, Savitree Racksas, Ballang Uppapong, Janisara Rudeeaneksin
Cureus.2024;[Epub] CrossRef - Risks for latent tuberculosis infection among health care workers in Indonesia
Darariani DARARIANI, Nur A. TABRI, Muhammad ILYAS, Syakib BAKRI, Rini R. BACHTIAR, Himawan D. SANUSI, Hasyim KASIM, Arifin SEWENG
Gazzetta Medica Italiana Archivio per le Scienze Mediche.2023;[Epub] CrossRef - Risk factors affecting anal fistula incidence: a single hospital study
Fadhli AZHIMI, Samuel SAMPETODING, M. Ihwan KUSUMA, Firdaus HAMID, Sachraswaty R. LAIDDING, Prihantono PRIHANTONO, Muhammad FARUK
Chirurgia.2023;[Epub] CrossRef - Managing an ageing healthcare workforce: a systematic literature review
Mari Kurashvili, Karin Reinhold, Marina Järvis
Journal of Health Organization and Management.2023; 37(1): 116. CrossRef - Latent tuberculosis infection (LTBI) in health-care workers: a cross-sectional study at a northern Peruvian hospital
Edinson Dante Meregildo-Rodriguez, Verónica Yuptón-Chávez, Martha Genara Asmat-Rubio, Gustavo Adolfo Vásquez-Tirado
Frontiers in Medicine.2023;[Epub] CrossRef - Changes in the rate of bacillus tuberculosis infection in health workers in the first year of the COVID-19 epidemic in Kashan- Iran
Mojgan Sehat, Reza Razzaghi, Mark Ghamsary, Monireh Faghir Ganji, Mojtaba Sehat
Heliyon.2023; 9(10): e20560. CrossRef
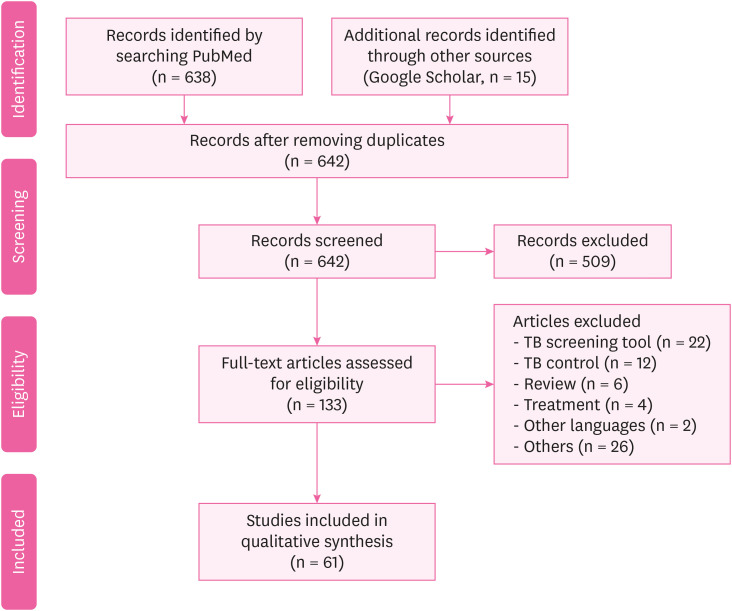
Fig. 1
| Number | Author | Publication year | Country | Study design | Participants | Tuberculosis assessment | Prevalence or incidence |
|---|---|---|---|---|---|---|---|
| 1 | Chen et al. [ | 2019 | China | Cross-sectional study | 487 | Questionnaire and IGRA for TB | IGRA positive: 33.9% |
| 2 | Nichimura et al. [ | 2018 | Japan | Prospective cohort study | 328 | IGRA for TB | HW: 0.149/100 person-years |
| Nursing student: 0.0825/100 person-years | |||||||
| 3 | Bukhary et al. [ | 2018 | Saudi Arabia | Cross-sectional study | 520 | IGRA for TB | QFT-GIT positive: 56/520 (10.8%) |
| 4 | Henderson et al. [ | 2017 | UK | Cross-sectional study | 587 | IGRA for TB | IGRA positive: 27% |
| 5 | Shrestha et al. [ | 2017 | Nepal | Cross-sectional study | 560 | Self-administered questionnaire and CXR | Direct contact with patients: 76.8% |
| Cough > 2 weeks: 10.4% | |||||||
| Chest radiography: 20.0% | |||||||
| 6 | Lacerda et al. [ | 2017 | Brazil | Cross-sectional study | 708 | Questionnaire and IGRA | LTBI prevalence: 27% (n = 196; 95% CI: 24%–31%) |
| 7 | Lacerda et al. [ | 2017 | Brazil | Cross-sectional study | 218 | TST | TST positive: 39.4% (95% CI: 32.9–45.9) and 54.1% (95% CI: 47.4–60.7) |
| 8 | Yoon et al. [ | 2017 | Korea | Cross-sectional study | 902 | TST, IGRA | TB patient care ≥ 1 year or more: 19.5% (176/902) |
| TST positive: 26.9% (243/902) | |||||||
| LTBI: 5.8% (52/902) | |||||||
| 9 | Janagond et al. [ | 2017 | India | Prospective cohort study | 206 | Questionnaires, TST | TST positive: 36.8% (76/206) |
| 10 | Napoli et al. [ | 2017 | Italy | Cross-sectional study | 2,290 | QFT, TST | TST positive: 141 (6.1%) |
| QFT positive: 16.4% | |||||||
| 11 | Gehanno et al. [ | 2017 | France | Retrospective study | 233,389 | Questionnaires | Nurse, health care assistants: 5.7/100,000 |
| Administrative staff: 1.27/100,000 | |||||||
| 12 | Pan et al. [ | 2015 | Taiwan | Matched cohort study | 44 | AFB staining | Smear positive: 26.8% (11/41) |
| Culture proven: 70.7% (29/41) | |||||||
| Pathologically diagnosed: 9.8% (4/41) | |||||||
| Clinically diagnosed: 24.4% (10/41) | |||||||
| 13 | He et al. [ | 2015 | China | Longitudinal study | 880 | TST, QFT | TST positive: 11.4% |
| QFT-GIT: 19.1% (OR: 142.62, 95% CI: 1.39–4.97) | |||||||
| BCG scar: OR: 0.53, 95% CI: 0.28–1.00 | |||||||
| 14 | Adams et al. [ | 2015 | South Africa | Prospective cohort study | 764 | TST, QFT, CXR | TST positive: 38% |
| QFT-GIT positive: 13%–22% | |||||||
| 15 | Hung et al. [ | 2015 | Taiwan | Prospective cross-sectional study | 193 | QFT-G, TST | TST positive: 88.8% |
| QFT-GIT positive: 14.5% | |||||||
| Multivariable logistic regression: only the QFT-G test was associated with age (35 years or greater) (adjusted OR: 2.53, p = 0.03) | |||||||
| 16 | Chen et al. [ | 2014 | China | Retrospective study | 996 | Sputum smear | TB: females > males (58.0% > 42.0%) |
| TB positive: males > females (88.5% > 83.4%, p = 0.031) | |||||||
| 17 | Garcell et al. [ | 2014 | Qatar | Cross-sectional study | 202 | TST, QFT-G | TST positive: 6.9% |
| QFT-G positive: 3.0% | |||||||
| TB positive is older than negative (44.5 vs. 38.9 years) | |||||||
| More experience as HWs (21.7 vs. 16.8 years) | |||||||
| Direct contact with Tb patients (83.3% vs. 25%) | |||||||
| 18 | Szep et al. [ | 2014 | USA | Cross-sectional study | 95 | TST | TST positive: 4.2% or 6.87 per 1,000 person weeks (95% CI: 1.87–17.60). |
| 19 | Muzzi et al. [ | 2014 | Italy | Cross-sectional study | 388 | TST | TST at T1 was positive: 11 (4.3%). ARTI was 1.6 (95% CI: 0.9–2.9) per 100 person-years |
| TST at T2 was positive: 9 (3.7%) | |||||||
| HWs PEARTI was 26 (95% CI 13.6–50) per 100 person-years | |||||||
| 20 | Wei et al. [ | 2013 | China | Observational study | 210 | IGRA, TST | QFT-GIT positive: 161 (76.7%) |
| QFT-GIT negative: 10 (19.01%) | |||||||
| Indeterminate results: 9 (4.3%) | |||||||
| (κ = 0.456, p < 0.001) | |||||||
| 21 | Gran et al. [ | 2013 | Norway | Cross-sectional study | 387 | QFT, TST | QFT-GIT positive: 3.4% |
| TST (≥ 6 mm): 214 (55.3%) | |||||||
| TST (≥ 15 mm): 53 (13.7%) | |||||||
| QFT, TST positive: 10 (4.7%) | |||||||
| 22 | Whitaker et al. [ | 2013 | Georgia | Prospective longitudinal study | 319 | TST, QFT-GIT | HWs from TB unit had higher prevalence of positive QFT-GIT and TST than those from non-TB unit: 107/194 (55%) vs. 30/125 (31%) QFT-GIT positive (p < 0.0001) and 128/189 (69%) vs. 64/119 (54%) TST positive (p = 0.01) |
| 23 | Kiertiburanakul et al. [ | 2012 | Thailand | Prospective study | 1,438 | TST | TST positive: 66.3% |
| TST conversion: 4.8 per 100 HCP-year | |||||||
| 9 (0.6%) HCP were diagnosed with active TB | |||||||
| 24 | Larcher et al. [ | 2012 | Italy | Cross-sectional study | 621 | TST, QFT | TST positive: 29.1% |
| QFT positive: 18.5% | |||||||
| 25 | Zwerling et al. [ | 2012 | Canada | Prospective longitudinal study | 388 | TST, QFT | TST positive: 5.7% (22/388, 95% CI: 3.6%–8.5%) |
| QFT positive: 6.2% (24/388, 95% CI: 4%–9.1%) | |||||||
| 26 | Borroto et al. [ | 2011 | Cuba | Cross-sectional study | 350 | TST | LTBI prevalence: 15.4%: it was highest in professionals (20.6%); 60.3% were non-reactors, and at the second test a year later 1.4% were converters |
| 27 | Moon et al. [ | 2011 | Korea | Cross-sectional study | 173 | TST, QFT | QFT-GIT positive: 21.4% |
| TST positive: 33.3% | |||||||
| κ = 0.234 | |||||||
| 28 | Sherman et al. [ | 2011 | Germany | Retrospective cohort study | 450 | TST | TST conversion: 93 |
| 29 | Kehinde et al. [ | 2011 | Nigeria | Descriptive study | 271 | Pre-tested questionnaire | AFB stain positive: 9 (3.3%) |
| Culture positive: 6 (2.2%) | |||||||
| The culture contamination: 1.8 per cent | |||||||
| 30 | Costa et al. [ | 2011 | Portugal | Cross-sectional study | 376 | QFT, TST | TST positive: 61 |
| 31 | Rafiza et al. [ | 2011 | Malaysia | Cross-sectional study | 954 | QFT, TST | The overall prevalence of latent tuberculosis infection among Health workers was 10.6% (CI: 8.6%–12.6%) |
| 32 | Park et al.[ | 2010 | Korea | Prospective study | 322 | QFT, TST | Both positive: 25 subjects (8%) |
| Follow-up after 1 year | |||||||
| QFT-GIT positive: between 3.3% and 5.7% | |||||||
| 33 | Cadmus et al. [ | 2010 | Nigeria | Retrospective study | 101 | AFB | AFB positive: 10 (13%) |
| 34 | Escombe et al. [ | 2010 | Peru | Cross-sectional study | 845 | QFT | QFT-GIT positive: 39 (56%) |
| 35 | Lambert et al. [ | 2012 | USA | Cross-sectional study | 200,744 | TST | TST positive: 6,049 (3%) |
| 36 | Schablon et al. [ | 2010 | Germany | Cross-sectional study | 2,028 | IGRA | QFT-GIT positive: 198 (9.9%) |
| TST positive: 480 (24.0%) | |||||||
| 37 | Jo et al. [ | 2013 | Korea | Cross-sectional study | 493 | TST, QFT | Doctors (n = 99): TST positive: 63 (41.4%)/QFT-GIT positive: 36 (23.7%) |
| Nurse (n = 168): TST positive: 119 (34.9%)/QFT-positive: 49 (14.4%) |
| Number | Author | Year | Country | Study design | Participants | Tuberculosis assessment | Estimate of risk |
|---|---|---|---|---|---|---|---|
| 1 | Wang et al. [ | 2018 | China | Cross-sectional study | 212 | Positive sputum acid-fast stains | TB: 760/100,000, RF: 51 years and above (aOR: 6.17, 95% CI: 1.35–28.28), being a nurse (aOR: 3.09, 95% CI: 1.15–8.32) |
| 2 | Kim et al. [ | 2017 | Korea | Prospective cohort study | 872 | TST, CXR | Age over 30 years: (p = 0.02), LTBI point prevalence: 6.6%, LTBI incidence: 2.4 per 100 HWs |
| 3 | Davidson et al. [ | 2017 | UK | Retrospective cohort study | 2,320 | TB surveillance, genotyping data | HWs: 23.4 (95% CI: 22.5–24.4), non-HWs: 16.2 (95% CI: 16.0–16.3) |
| 4 | Belo et al. [ | 2017 | Mozambique | Cross-sectional study | 316 | Symptom screening questionnaire | LTBI: 34.4%, working > 8 years: 39.3%, no BCG vaccine: 39.6%, immunocompromised: 78.1% |
| 5 | Du et al. [ | 2017 | China | Cross-sectional study | 186 | Questionnaires | Medical professionals (PR = 2.40), laboratory technicians (PR = 2.17), other hospital staff (PR = 1.04) |
| 6 | Bonini et al. [ | 2017 | Italy | Cross-sectional study | 580 | Questionnaires, TST | Previous BCG vaccination: OR: 344, CI: 43.72–2,718.41, p < 0.001, origin in high-risk countries: OR: 401.68, CI: 50.60–3,188.69, p < 0.001 |
| 7 | Weng et al. [ | 2016 | Swaziland | Cross-sectional study | 186 | Questionnaires | Nurses (OR: 39.87, 95% CI: 2.721–584.3), other HWs (OR: 99.34, 95% CI: 7.469–1,321) |
| 8 | Nonghanphithak et al. [ | 2016 | Thailand | Cross-sectional study | 112 | QFT, questionnaires | Age ≥ 30 years (OR: 18.88, 95% CI: 1.52–234.36), nurse (OR: 2.78, 95% CI: 1.19–6.49), job for ≥ 10 years (OR: 8.78, 95% CI: 1.26–61.29) |
| 9 | Tudor et al. [ | 2016 | South Africa | Case-control study | 307 | Questionnaires | HWs living with HIV (OR: 6.35, 95% CI: 3.54–11.37) spent time working in areas with patients (OR: 2.24; 95% CI: 1.40–3.59) |
| 10 | Ito et al. [ | 2016 | Japan | Retrospective study | 875 | IGRA, CXR | Multivariate analysis (OR: 8.2, 95% CI: 1.3–78.3, p = 0.03), longer duration of contact (> 7 days, 12/12 [100%], vs. ≤ 7 days, 18/43 [41.9%]; p = 0.0002), fewer symptoms (> 7 days, 5/12 [41.7%] vs. ≤ 7 days, 35/43 [81.4%]; p = 0.01). |
| 11 | Tsang et al. [ | 2015 | Hong Kong | Prospective cohort study | 279 | IGRA, QFT | QFT-GIT positive: (exposed: 19.5%, non-exposed: 20.8%, RR = 0.96, 95% CI: 0.74–1.25, p > 0.05) |
| 12 | Agaya et al. [ | 2015 | Kenya | Cross-sectional survey | 1,416 | Standardized questionnaire | LTBI prevalence: (p = 0.72), work year: p < 0.01 |
| 13 | McCarthy et al. [ | 2015 | South Africa | Cross-sectional study | 199 | IGRA, TST | Incident LTBI (IGRA): 25/97 (26%; incident rate 29 cases/100 person-years, 95% CI: 20–44), TST: 25/93 (27%; incident rate 29 cases/100 person-years, 95% CI: 19–42) |
| 14 | Rutanga et al. [ | 2015 | Rwanda | Cross-sectional study | 1,131 | TST | LTBI prevalence: (62.1%), TST positive odds TST: 2.71 times greater (95% CI: 2.01–3.67), work year odds: increasing 4% (aOR: 1.04, 95% CI: 1.02%–1.05%) per year |
| 15 | Chu et al. [ | 2014 | Taiwan | Population-based cohort study | 11,811 | Chart review | TB incidence: HWs vs matched subjects (61.08 vs. 37.81 per 100,000 person-years) |
| Risk of TB: HWs (aHR: 1.62, 95% CI: 1.08–2.43) | |||||||
| 16 | Tudor et al. [ | 2014 | South Africa | Retrospective cohort study | 1,313 | Chart review | HWs living with HIV had a greater incidence of TB (IRR: 3.2, 95% CI: 1.54–6.66) than HIV-negative HWs |
| 17 | Zhou et al. [ | 2014 | China | Cross-sectional study | 712 | TST | TB hospital: 58.0% (n = 127), non-TB hospital: 33.9% (n = 105) (OR: 2.40, 95% CI:1.59–3.62), (6–10 years vs. ≤ 5 years [OR: 1.89, 95% CI: 1.10–3.25] and > 10 vs. ≤ 5 [OR:1.80; 95% CI: 1.20–2.68]) |
| 18 | Claassens et al. [ | 2013 | South Africa | Cross-sectional study | 133 | Sputum smear | The infection control audit score: OR: 1.04, 95% CI: 1.01–1.08, p = 0.02, the number of staff: OR: 3.78, 95% CI: 1.77–8.08, the number of staff remained: OR: 3.33, 95% CI: 1.37–8.08 |
| 19 | Durando et al. [ | 2013 | Italy | Cross-sectional study | 881 | TST | Born in high TB incidence areas (≥ 20 cases per 100,000 population) |
| 20 | Kim et al. [ | 2013 | Korea | Cross-sectional study | 2,132 | TST | TST positive: 778 (36.5%), being older (OR: 1.10, 95% CI: 1.06–1.13, p < 0.001), male (OR: 1.78, 95% CI: 1.21–2.62, p = 0.003), re-joining the hospital workforce (OR: 1.58, 95% CI: 1.04–2.40, p = 0.032) |
| 21 | Casas et al. [ | 2013 | Spain | Cohort analysis | 614 | TST | High risk worker hazard ratio: 1.55 (95% CI: 1.05–2.27) gender, age and professional status |
| 22 | Mathew et al. [ | 2013 | South India | Case-control study | 101 | TST | BMI < 19 kg/m2 (OR: 2.96, 95% CI: 1.49–5.87), contact with patients (OR: 2.83, 95% CI: 1.47–5.45), being employed in medical wards (OR: 12.37, 95% CI: 1.38–110.17), microbiology laboratories (OR: 5.65, 95% CI: 1.74–18.36) |
| 23 | He et al. [ | 2012 | China | Cross-sectional study | 999 | TST, QFT-GIT | QFT-GIT-positive: 683 (68%) associated with greater age, longer HW career, TB disease in a co-worker and greater daily patient exposure using multivariable analysis |
TB: tuberculosis; HW: health worker; IGRA: interferon-gamma releasing assay; QFT-GIT: QuantiFERON-TB Gold-In-Tube; CXR: chest X-ray; LTBI: latent TB infection; CI: confidence interval; TST: tuberculin skin test; QFT: QuantiFERON-TB; AFB: acid fast bacilli; BCG: Bacillus Calmette-Guerin; QFT-G: QuantiFERON-TB Gold; PEARTI: post-exposure rate of tuberculosis infection.
TB: tuberculosis; HW: health worker; RF: risk factor; aOR: adjusted odds ratio; CI: confidence interval; TST: tuberculin skin test; CXR: chest X-ray; LTBI: latent tuberculosis infection; BCG: Bacillus Calmette-Guerin; PR: prevalence ratio; OR: odds ratio; QFT: QuantiFERON-TB; HIV: human immunodeficiency virus; IGRA: interferon-gamma releasing assay; QFT-GIT: QuantiFERON-TB Gold-In-Tube; RR: risk ratio; BMI: body mass index.
 KSOEM
KSOEM

 Cite
Cite

