Articles
- Page Path
- HOME > Ann Occup Environ Med > Volume 29; 2017 > Article
- Research Article Levels of blood lead and urinary cadmium in industrial complex residents in Ulsan
- Sang Hoon Kim, Yang Ho Kim, Hyun Chan An, Joo Hyun Sung, Chang Sun Sim
-
Annals of Occupational and Environmental Medicine 2017;29:26.
DOI: https://doi.org/10.1186/s40557-017-0179-7
Published online: June 26, 2017
Department of Occupational and Environmental Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, 877, Bangeojinsunhwando-ro, Dong-gu, Ulsan, 44033 Republic of Korea
© The Author(s). 2017
Open AccessThis article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Abstract
-
Background Populations neighboring industrial complexes are at an increased health risk, due to constant exposure to various potentially hazardous compounds released during industrial production activity. Although there are many previous studies that focus on occupational exposure to heavy metals, studies that focused on environmental exposure to lead and cadmium are relatively rare. The purpose of this study is to evaluate the extent of the environmental exposure of heavy metals in residents of industrial area.
-
Methods Four areas in close proximity to the Ulsan petrochemical industrial complex and the Onsan national industrial complex were selected to be included in the exposure group, and an area remotely located from these industrial complexes was selected as the non-exposure group. Among the residents of our study areas, a total of 1573 subjects aged 20 years and older were selected and all study subjects completed a written questionnaire. Blood and urine samples were obtained from about one third of the subjects (465 subjects) who provided informed consent for biological sample collection. Total 429 subjects (320 subjects from exposure area, 109 subjects from non-exposure area) were included in final analysis.
-
Results The geometric mean blood lead level among the subjects in the exposed group was 2.449 μg/dL, which was significantly higher than the non-exposure group’s level of 2.172 μg/dL. Similarly, the geometric mean urine cadmium levels between the two groups differed significantly, at 1.077 μg/g Cr. for the exposed group, and 0.709 μg/g Cr. for the non-exposure group.In a multiple linear regression analysis to determine the relationship between blood lead level and related factors, the results showed that blood lead level had a significant positive correlation with age, the male, exposure area, and non-drinkers. In the same way, urine cadmium level was positively correlated with age, the female, exposure area, and smokers.
-
Conclusions This study found that blood lead levels and urine cadmium levels were significantly higher among the residents of industrial areas than among the non-exposure area residents, which is thought to be due to the difference in environmental exposure of lead and cadmium. Furthermore, it was clear that at a low level of exposure, differences in blood lead or urine cadmium levels based on age, gender, and smoking status were greater than the differences based on area of residence. Therefore, when evaluating heavy metal levels in the body at a low level of exposure, age, gender, and smoking status must be adjusted, as they are significant confounding factors.
Background
Methods
Results
Discussion
Conclusions
Acknowledgements
Abbreviations
AM
BMI
GF-AAS
GM
KNEHS
ln
NHANES
- 1. Nadal M, Schuhmacher M, Domingo JL. Long-term environmental monitoring of persistent organic pollutants and metals in a chemical/petrochemical area: human health risks. Environ Pollut 2011;159:1769–1777. 10.1016/j.envpol.2011.04.007. 21536358.ArticlePubMed
- 2. Duruibe J, Ogwuegbu M, Egwurugwu J. Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2007;2:112–118.
- 3. Hong CD, Hanenson IB, Lerner S, Hammond PB, Pesce AJ, Pollak VE. Occupational exposure to lead: effects on renal function. Kidney Int 1980;18:489–494. 10.1038/ki.1980.162. 7230612.ArticlePubMed
- 4. Blando J, Lu SE, Gu H, Lin Y, Marshall EG. Variability and trend of multiple blood lead measures among construction and manufacturing workers. Occup Environ Med 2013;70:774–781. 10.1136/oemed-2013-101370. 23839661.ArticlePubMed
- 5. McDiarmid MA, Freeman CS, Grossman EA, Martonik J. Biological monitoring results for cadmium exposed workers. Am Ind Hyg Assoc J 1996;57:1019–1023. 10.1080/15428119691014350. 8931310.ArticlePubMed
- 6. Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, environmental exposure, and health outcomes. Environ Health Perspect 2010;118:182–190. 10.1289/ehp.0901234. 20123617.ArticlePubMedPMC
- 7. Tong S, Von Schirnding YE, Prapamontol T. Environmental lead exposure: a public health problem with global dimensions. Servir (Lisbon, Portugal) 2000;49:35–43.PubMed
- 8. Abadin H, et al. Toxicology profile for lead. 2007.
- 9. Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Guallar E. Cadmium exposure and hypertension in the 1999-2004 national health and nutrition examination survey (nhanes). Environ Health Perspect 2008;116:51–56. 10.1289/ehp.10764. 18197299.ArticlePubMed
- 10. https://ntp.niehs.nih.gov/ntp/ohat/lead/final/leadappendixe_final_508.pdf.
- 11. Yu CC, Lin JL, Lin-Tan DT. Environmental exposure to lead and progression of chronic renal diseases: a four-year prospective longitudinal study. J Am Soc Nephrol 2004;15:1016–1022. 10.1097/01.ASN.0000118529.01681.4F. 15034104.PubMed
- 12. Gambelunghe A, et al. Low-level exposure to lead, blood pressure, and hypertension in a population-based cohort. Environ Res 2016;149:157–163. 10.1016/j.envres.2016.05.015. 27208466.ArticlePubMed
- 13. Kordas K, et al. Deficits in cognitive function and achievement in Mexican first-graders with low blood lead concentrations. Environ Res 2006;100:371–386. 10.1016/j.envres.2005.07.007. 16169549.ArticlePubMed
- 14. Alfvén T, Järup L, Elinder CG. Cadmium and lead in blood in relation to low bone mineral density and tubular proteinuria. Environ Health Perspect 2002;110:699. 10.1289/ehp.02110699. 12117647.Article
- 15. Ferraro PM, et al. Low level exposure to cadmium increases the risk of chronic kidney disease: analysis of the NHANES 1999-2006. BMC Public Health 2010;10:1. 10.1186/1471-2458-10-304. 20043862.ArticlePubMedPMCPDF
- 16. Järup L, et al. Low level exposure to cadmium and early kidney damage: the OSCAR study. Occup Environ Med 2000;57:668–672. 10.1136/oem.57.10.668. 10984338.ArticlePubMedPMC
- 17. Järup L, Alfvén T. Low level cadmium exposure, renal and bone effects-the OSCAR study. Biometals 2004;17:505–509. 10.1023/B:BIOM.0000045729.68774.a1. 15688854.ArticlePubMed
- 18. https://www.atsdr.cdc.gov/toxprofiles/tp5.pdf.
- 19. Parsons PJ, Slavin W. A rapid Zeeman graphite furnace atomic absorption spectrometric method for the determination of lead in blood. Spectrochim Acta B 1993;48:925–939. 10.1016/0584-8547(93)80094-B.Article
- 20. Subramanian KS, Meranger JC, MacKeen JE. Graphite furnace atomic absorption spectrometry with matrix modification for determination of cadmium and lead in human urine. Anal Chem 1983;55:1064–1067. 10.1021/ac00258a020. 6881524.ArticlePubMed
- 21. Muntner P, et al. Continued decline in blood lead levels among adults in the United States: the National Health and nutrition examination surveys. Arch Intern Med 2005;165:2155–2161. 10.1001/archinte.165.18.2155. 16217007.ArticlePubMed
- 22. Velea T, et al. Heavy metal contamination in the vicinity of an industrial area near Bucharest. Environ Sci Pollut Res 2009;16:27–32. 10.1007/s11356-008-0073-5.ArticlePDF
- 23. https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Jul2014.pdf.
- 24. Moon CS, et al. Dietary intake of cadmium and lead among the general population in Korea. Environ Res 1995;71(1):46–54. 10.1006/enrs.1995.1066. 8757238.ArticlePubMed
- 25. Jarup L, et al. Health effects of cadmium exposure--a review of the literature and a risk estimate. Scand J Work Environ Health 1998;24(Suppl 1):1–51. 9569444.
- 26. Brockhaus A, et al. Levels of cadmium and lead in blood in relation to smoking, sex, occupation, and other factors in an adult population of the FRG. Int Arch Occup Environ Health 1983;52:167–175. 10.1007/BF00405420. 6629506.ArticlePubMedPDF
- 27. Brody DJ, et al. Blood lead levels in the US population: phase 1 of the third National Health and nutrition examination survey (NHANES III, 1988 to 1991). JAMA 1994;272:277–283. 10.1001/jama.1994.03520040039038. 8028140.ArticlePubMed
- 28. Rabinowitz MB. Toxicokinetics of bone lead. Environ Health Perspect 1991;91:33. 10.1289/ehp.919133. 2040248.ArticlePubMedPMC
- 29. Godt J, et al. The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol 2006;1:1. 10.1186/1745-6673-1-22.ArticlePMCPDF
- 30. Lee BK, Kim Y. Sex-specific profiles of blood metal levels associated with metal–iron interactions. Saf Health Work 2014;5:113–117. 10.1016/j.shaw.2014.06.005. 25379323.ArticlePubMedPMC
- 31. Olsson M, et al. Cadmium in blood and urine--impact of sex, age, dietary intake, iron status, and former smoking--association of renal effects. Environ Health Perspect 2002;110:1185. 10.1289/ehp.021101185. 12460796.ArticlePubMedPMC
- 32. Vahter M, et al. Gender differences in the disposition and toxicity of metals. Environ Res 2007;104:85–95. 10.1016/j.envres.2006.08.003. 16996054.ArticlePubMed
- 33. Berglund M, et al. Intestinal absorption of dietary cadmium in women depends on body iron stores and fiber intake. Environ Health Perspect 1994;102:1058. 10.1289/ehp.941021058. 7713018.ArticlePubMedPMC
- 34. Gallagher CM, Chen JJ, Kovach JS. The relationship between body iron stores and blood and urine cadmium concentrations in US never-smoking, non-pregnant women aged 20–49 years. Environ Res 2011;111:702–707. 10.1016/j.envres.2011.03.007. 21507392.ArticlePubMed
- 35. Lewis G, et al. Contribution of cigarette smoking to cadmium accumulation in man. Lancet 1972;299:291–292. 10.1016/S0140-6736(72)90294-2.Article
- 36. Jung M, Thornton I, Chon H. Arsenic, cadmium, copper, lead, and zinc concentrations in cigarettes produced in Korea and the United Kingdom. Environ Technol 1998;19:237–241. 10.1080/09593331908616676.Article
REFERENCES
Figure & Data
REFERENCES
Citations

- Spatial distributions, sources, and ecological risks of metals in soils from the largest industrial city of Ulsan, South Korea
In-Gyu Cho, Sung-Deuk Choi, Leesun Kim, Min-Kyu Park, Sung-Eun Lee
Environmental Geochemistry and Health.2026;[Epub] CrossRef - Cadmium and lead in occupationally exposed e-waste workers: Health implications
Olubayo Michael Akinosun, Ayobola Abimbola Sonuga, Bukola Fasilat Ayoola, Oyebola Oluwagbemiga Sonuga
Toxicology and Industrial Health.2026;[Epub] CrossRef - Heavy metals and ocular toxicity: Breakthroughs in accumulation mechanisms, molecular pathways, and cutting-edge therapeutic strategies
Lixin Li, Quanyong Yi, Ziya Ma, Chengyan Fang, Ji Yang, Hai Liu, Ping Xiang
Environmental Chemistry and Ecotoxicology.2025; 7: 2395. CrossRef - Exploring the association between metal(loid)s and human semen quality: a preliminary case study in a petrochemical complex
Elena Sánchez-Resino, Ana González-Ruiz, Jordi Sierra, Carlos Martínez-Pinto, María Fernández de la Puente, Nadine Alkhoury, María Ángeles Martínez, Nancy Babio, Albert Salas-Huetos, Jordi Salas-Salvadó, Rubén Gil-Solsona, Pablo Gago-Ferrero, José L. Domi
Environmental Science and Pollution Research.2025; 32(46): 26207. CrossRef - Simultaneous comparison of the chemical composition and attributable source of PM2.5 during 2014–2018 in major metropolitan cities in South Korea: impacts of policy interventions
Sangcheol Kim, Seung-Muk Yi, Jung Min Park, In Ho Song, Kwonho Jeon, Jieun Park
Environmental Research Letters.2024; 19(11): 114020. CrossRef - Environmental pollution by xenobiotics as a risk factor for the development of reproductive complications in the population of the industrial region
T.A. Holovkova
Hygiene of Populated Places.2024; 2024(74): 162. CrossRef - Investigating Blood Lead Levels and Its Health Effects on Employees of a Petroleum Industry and the Surrounding Residents
Luay M. Mohammad, Manoochehr Karami, Yadollah Mehrabi, Seyed Saeed Hashemi Nazari, Somayeh Farhang Dehghan, Hasan A. Baiee, Mohammed Rafiee
Journal of Occupational & Environmental Medicine.2024; 66(11): 924. CrossRef - Assessment of heavy radionuclides in blood samples for workers of a cement factory by X-ray fluorescence
Zakariya A. Hussein
Journal of Radiation Research and Applied Sciences.2023; 16(2): 100553. CrossRef - Urinary concentrations of heavy metals in pregnant women living near a petrochemical area according to the industrial activity
Xiruo Kou, Lucía Iglesias-Vázquez, Martí Nadal, Josep Basora, Victoria Arija
Environmental Research.2023; 235: 116677. CrossRef - The Protection of Zinc against Acute Cadmium Exposure: A Morphological and Molecular Study on a BBB In Vitro Model
Jacopo J. V. Branca, Donatello Carrino, Ferdinando Paternostro, Gabriele Morucci, Claudia Fiorillo, Claudio Nicoletti, Massimo Gulisano, Carla Ghelardini, Lorenzo Di Cesare Mannelli, Matteo Becatti, Alessandra Pacini
Cells.2022; 11(10): 1646. CrossRef - Relationship between renal function and metal exposure of residents living near the No. 6 Naphtha Cracking Complex: A cross-sectional study
Tzu-Hsuen Yuan, Ming-Jie Jhuang, Yen-Po Yeh, Yi-Hsuan Chen, Sasha Lu, Chang-Chuan Chan
Journal of the Formosan Medical Association.2021; 120(10): 1845. CrossRef - Blood lead level and Helicobacter pylori infection in a healthy population: A cross-sectional study
Won-Ju Park, Soo-Hyeon Kim, WonYang Kang, Ji-Sung Ahn, Seunghyeon Cho, Dae-Young Lim, Suwhan Kim, Jai-Dong Moon
Archives of Environmental & Occupational Health.2020; 75(6): 333. CrossRef - Lead and kidney: Concentrations, variabilities, and associations across the various stages of glomerular function
Ram B. Jain
Journal of Trace Elements in Medicine and Biology.2019; 54: 36. CrossRef - Human Blood Lead Levels and the First Evidence of Environmental Exposure to Industrial Pollutants in the Amazon
Thaís Karolina Lisboa de Queiroz, Karytta Sousa Naka, Lorena de Cássia dos Santos Mendes, Brenda Natasha Souza Costa, Iracina Maura de Jesus, Volney de Magalhães Câmara, Marcelo de Oliveira Lima
International Journal of Environmental Research and Public Health.2019; 16(17): 3047. CrossRef - A study on the concentration of biomarkers for heavy metals and VOCs in the residents living in the vicinity of Gwangyang Industrial Complex in Korea
Kyoungho Lee, Seokwon Lee, Ryoungme Ahn, Jae Hyoun Kim, Bu-Soon Son
Journal of Odor and Indoor Environment.2019; 18(3): 228. CrossRef - Risk assessment of lead and cadmium exposure from electronic waste recycling facilities in Southern Thailand
Peeranart Kiddee, Somsiri Decharat
Environmental Earth Sciences.2018;[Epub] CrossRef - Comparison of a 10-Year Cumulative Age-Standardized Incidence Rate of Lung Cancer among Metropolitan Cities in Korea (During the 2000–2009 Period): Review of Occupational and Environmental Hazards Associated with Lung Cancer
Joo Hyun Sung, Chang Sun Sim, Minsu Ock, Inbo Oh, Kyoung Sook Jeong, Cheolin Yoo
International Journal of Environmental Research and Public Health.2018; 15(6): 1259. CrossRef - Environmental and Body Concentrations of Heavy Metals at Sites Near and Distant from Industrial Complexes in Ulsan, Korea
Joo Hyun Sung, Inbo Oh, Ahra Kim, Jiho Lee, Chang Sun Sim, Cheolin Yoo, Sang Jin Park, Geun-Bae Kim, Yangho Kim
Journal of Korean Medical Science.2018;[Epub] CrossRef
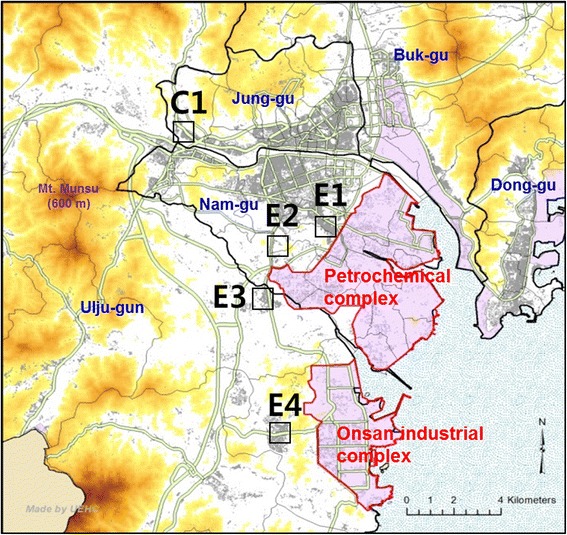
Fig. 1
| Non-exposure | Exposure | p value | ||
|---|---|---|---|---|
| Gender | Men | 43 | 130 | 0.829 |
| Women | 66 | 190 | ||
| Mean age (AM) | 48.08 ± 17.17 | 51.41 ± 16.32 | 0.070 | |
| Age group | 20–29 | 19 | 42 | 0.483 |
| 30–39 | 17 | 35 | ||
| 40–49 | 23 | 62 | ||
| 50–59 | 17 | 66 | ||
| 60–69 | 20 | 68 | ||
| Over 70 | 13 | 47 | ||
| Body Mass Index | Non-obese | 79 | 227 | 0.759 |
| Obese | 30 | 93 | ||
| Smoking status | Non-smoker | 73 | 227 | 0.436 |
| Smoker | 36 | 93 | ||
| Drinking status | Non-drinker | 74 | 202 | 0.370 |
| Drinker | 35 | 118 | ||
| Educational status | High school and less | 49 | 214 | <0.001* |
| College and more | 60 | 106 | ||
| Distance to road | Above 100 m | 59 | 143 | 0.098 |
| Under 100 m | 50 | 175 | ||
| Classification variables | Number | Lead | Cadmium | |||||
|---|---|---|---|---|---|---|---|---|
| Total | Non-exposure | Exposure | Total | Non-exposure | Exposure | |||
| 2.172 ± 1.590 | 2.449 ± 1.510* | 0.709 ± 2.344 | 1.077 ± 2.169* | |||||
| Gender | Men | 173 | 2.835 ± 1.516 (2.663–3.018) | 2.599 ± 1.486 | 2.917 ± 1.522 | 0.718 ± 2.221 (0..603–0.810) | 0.484 ± 2.169 | 0.818 ± 2.149* |
| Women | 256 | 2.108 ± 1.484 (2.008–2.213)* | 1.933 ± 1.600 | 2.173 ± 1.436* | 1.185 ± 2.144 (1.079–1.302)* | 0.908 ± 2.254 | 1.300 ± 2.063* | |
| Age group | 20–29 | 61 | 1.698 ± 1.450 (1.544–1.868) | 1.544 ± 1.516 | 1.773 ± 1.413 | 0.373 ± 1.966 (0.313–0.443) | 0.275 ± 2.169 | 0.428 ± 1.797* |
| 30–39 | 42 | 2.139 ± 1.404 (1.946–2.351) † | 2.247 ± 1.489 | 2.088 ± 1.364 | 0.638 ± 1.869 (0.536–0.759) † | 0.535 ± 2.112 | 0.695 ± 1.730 | |
| 40–49 | 85 | 2.386 ± 1.444 (2.204–2.583) † | 2.283 ± 1.516 | 2.426 ± 1.419 | 0.806 ± 2.043 (0.691–0.941) † | 0.690 ± 2.127 | 0.855 ± 2.007 | |
| 50–59 | 83 | 2.683 ± 1.573 (2.430–2.962) †§ | 2.434 ± 2.751 | 2.751 ± 1.610 | 1.367 ± 1.830 (1.198–1.560) †§ | 0.983 ± 1.826 | 1.488 ± 1.785* | |
| 60–69 | 88 | 2.594 ± 1.467 (2.391–2.814) †§ | 2.420 ± 1.424 | 2.647 ± 1.480 | 1.453 ± 1.872 (1.272–1.660) †§ | 1.165 ± 1.840 | 1.550 ± 1.862 | |
| Over 70 | 60 | 2.701 ± 1.604 (2.390–3.052) †§ | 2.290 ± 2.117 | 2.827 ± 1.434 | 1.628 ± 1.826 (1.394–1.903) †§ | 1.198 ± 1.704 | 1.734 ± 1.841 | |
| Body Mass Index | Non-obese (<25 kg/m2) | 306 | 2.319 ± 1.543 (2.208–2.435) | 1.977 ± 1.599 | 2.451 ± 1.502* | 0.970 ± 2.262 (0.885–1.064) | 0.687 ± 2.351 | 1.094 ± 2.157* |
| Obese (≥25 kg/m2) | 123 | 2.522 ± 1.509 (2.344–2.715) | 2.784 ± 1.417 | 2.443 ± 1.532 | 0.964 ± 2.254 (0.834–1.115) | 0.768 ± 2.347 | 1.038 ± 2.204 | |
| Smoking status | Non-smoker | 300 | 2.179 ± 1.517 (2.078–2.284) | 1.906 ± 1.571 | 2.274 ± 1.486* | 1.027 ± 2.337 (0.933–1.131) | 0.752 ± 2.496 | 1.136 ± 2.233* |
| Smoker | 129 | 2.905 ± 1.474 (2.715–3.108)* | 2.831 ± 1.446 | 2.934 ± 1.487 | 0.844 ± 2.038 (0.746–0.956)* | 0.628 ± 2.023 | 0.946 ± 1.983* | |
| Drinking status | Non-drinker | 276 | 2.476 ± 1.545 (2.351–2.607) | 2.332 ± 1.538 | 2.531 ± 1.546 | 0.812 ± 2.225 (0.739–0.893) | 0.595 ± 2.259 | 0.911 ± 2.149* |
| Drinker | 153 | 2.205 ± 1.503 (2.066–2.353)* | 1.870 ± 1.652 | 2.315 ± 1.439* | 1.330 ± 2.103 (1.181–1.498)* | 1.027 ± 2.269 | 1.436 ± 2.023* | |
| Educational status | High school and less | 263 | 2.514 ± 1.500 (2.393–2.641) | 2.314 ± 1.654 | 2.562 ± 1.461 | 1.299 ± 2.033 (1.191–1.415) | 1.068 ± 1.904 | 1.358 ± 2.049* |
| College and more | 166 | 2.172 ± 1.567 (2.027–2.327)* | 2.063 ± 1.532 | 2.236 ± 1.586 | 0.608 ± 2.122 (0.542–0.683)* | 0.507 ± 2.360 | 0.675 ± 1.950* | |
| Distance to road | Above 100 m | 202 | 2.320 ± 1.538 (2.185–2.463) | 2.167 ± 1.502 | 2.386 ± 1.550 | 0.944 ± 2.231 (0.845–1.055) | 0.686 ± 2.452 | 1.077 ± 2.062* |
| Under 100 m | 225 | 2.422 ± 1.533 (2.290–2.562) | 2.179 ± 1.695 | 2.497 ± 1.477* | 0.992 ± 2.290 (0.889–1.106) | 0.736 ± 2.232 | 1.080 ± 2.267* | |
| Independent variables | β coefficient (Standardized β coefficient) | p value | Model | |
|---|---|---|---|---|
| R2 | p value | |||
| Age (Years) | 0.009 (0.363) | <0.001 | 0.260 | <0.001 |
| Education level (College and more vs. High school and less) | −0.023 (−0.026) | 0.619 | ||
| Drinking status (Yes vs. No) | −0.144 (−0.161) | <0.001 | ||
| Smoking status (Yes vs. No) | 0.081 (0.086) | 0.159 | ||
| Gender (Men vs. Women) | 0.198 (0.227) | <0.001 | ||
| Exposure (Yes vs. No) | 0.088 (0.090) | 0.039 | ||
| Distance to road (Under 100 m vs. Above 100 m) | 0.014 (0.016) | 0.711 | ||
| Independent variables | β coefficient (Standardized β coefficient) | p value | Model | ||
|---|---|---|---|---|---|
| R2 | p value | ||||
| Total | Age (Years) | 0.026 (0.531) | <0.001 | 0.480 | <0.001 |
| (n = 429) | Education level (College and more vs. High school and less) | −0.106 (−0.064) | 0.153 | ||
| Drinking status (Yes vs. No) | −0.029 (−0.017) | 0.679 | |||
| Smoking status (Yes vs. No) | 0.261 (0.147) | 0.004 | |||
| Gender (Men vs. Women) | −0.656 (−0.396) | <0.001 | |||
| Exposure (Yes vs. No) | 0.328 (0.175) | <0.001 | |||
| Smoker | Age (Years) | 0.025 (0.608) | <0.001 | 0.521 | <0.001 |
| (n = 129) | Education level (College and more vs. High school and less) | −0.162 (−0.113) | 0.124 | ||
| Drinking status (Yes vs. No) | −0.238 (−0.113) | 0.085 | |||
| Gender (Men vs. Women) | −0.444 (−0.159) | 0.014 | |||
| Exposure (Yes vs. No) | 0.427 (0.270) | <0.001 | |||
| Non-smoker | Age (Years) | 0.027 (0.520) | <0.001 | 0.468 | <0.001 |
| (n = 300) | Education level (College and more vs. High school and less) | −0.054 (−0.031) | 0.584 | ||
| Drinking status (Yes vs. No) | 0.009 (0.005) | 0.914 | |||
| Gender (Men vs. Women) | −0.706 (−0.318) | <0.001 | |||
| Exposure (Yes vs. No) | 0.284 (0.144) | 0.001 | |||
unit: arithmetic mean ± standard deviation
*
unit: geometric mean ± standard deviation (95% confidence interval)
*
†
§
 KSOEM
KSOEM

 Cite
Cite