Articles
- Page Path
- HOME > Ann Occup Environ Med > Volume 33; 2021 > Article
- Research Article Association between prenatal polycyclic aromatic hydrocarbons and infantile allergic diseases modified by maternal glutathione S-transferase polymorphisms: results from the MOCEH birth cohort
-
Tai Kyung Koh1,2
 , Hyesook Park2,3
, Hyesook Park2,3 , Yun-Chul Hong4
, Yun-Chul Hong4 , Mina Ha5
, Mina Ha5 , Yangho Kim6
, Yangho Kim6 , Bo-Eun Lee7
, Bo-Eun Lee7 , Surabhi Shah8
, Surabhi Shah8 , Eunhee Ha2,8
, Eunhee Ha2,8
-
Annals of Occupational and Environmental Medicine 2021;33:e12.
DOI: https://doi.org/10.35371/aoem.2021.33.e12
Published online: April 23, 2021
1Department of Occupational and Environmental Medicine, Ewha Womans University Mokdong Hospital, Seoul, Korea.
2Graduate Program in System Health Science and Engineering, Ewha Womans University, Seoul, Korea.
3Department of Preventive Medicine, College of Medicine, Ewha Womans University, Seoul, Korea.
4Department of Preventive Medicine, College of Medicine, Seoul National University, Seoul, Korea.
5Department of Preventive Medicine, Dankook University College of Medicine, Cheonan, Korea.
6Department of Occupational and Environmental Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea.
7Environmental Health Research Department, National Institute of Environmental Research, Incheon, Korea.
8Department of Occupational and Environmental Medicine, College of Medicine, Ewha Womans University, Seoul, Korea.
- Correspondence: Surabhi Shah. Department of Occupational and Environmental Medicine, College of Medicine, Ewha Womans University, 25 Magokdong-ro 2-gil, Gangseo-gu, Seoul 07804, Korea. surabhi.3007@gmail.com
- Correspondence: Eunhee Ha. Department of Occupational and Environmental Medicine, College of Medicine, Ewha Womans University, 25 Magokdong-ro 2-gil, Gangseo-gu, Seoul 07804, Korea. eunheeha@ewha.ac.kr
- *Equal contribution as co-corresponding authors.
Copyright © 2021 Korean Society of Occupational & Environmental Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (https://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background Prenatal exposure to polycyclic aromatic hydrocarbons (PAH) has been linked to allergic disease onset. Variations in the glutathione S-transferase (GST) gene family can impact the progression of allergic diseases. We sought to examine the association between prenatal PAH exposure and infantile allergic diseases in 6-month-old infants, and how maternal glutathione S-transferase M1 (GSTM1) or T1 (GSTT1) polymorphism affects the association between prenatal PAH exposure and allergic diseases in the Mothers and Children's Environmental Health (MOCEH) study.
-
Methods The study sample comprised 349 infants and their mothers from the MOCEH study, for whom 1-hydroxypyrene (1-OHP) and 2-naphthol were measured in both the early period of pregnancy and late period of pregnancy. An infant was deemed to be affected by an allergic disease if diagnosed with or if developed at least one of the allergic diseases. A logistic regression analysis was performed to study the association between urinary 1-OHP and 2-naphthol levels during pregnancy and allergic diseases in 6-month-old infants. Furthermore, analyses stratified by maternal GSTM1 or GSTT1 present/null polymorphisms were performed.
-
Results The risk of allergic diseases in 6-month-old infants was significantly increased in accordance with an increase in urinary 1-OHP during the early period of pregnancy (odds ratio [OR]: 1.84; 95% confidence interval [CI]: 1.05, 3.23; by one log-transformed unit of 1-OHP μg/g creatinine). The increased risk of infantile allergic diseases associated with urinary 1-OHP during the early period of pregnancy was limited to the maternal GSTT1 null type (OR: 2.69; 95% CI: 1.17, 6.21, by one log-transformed unit of 1-OHP μg/g creatinine); however, the Relative Excess Risk due to Interaction was not statistically significant.
-
Conclusions The present study found that infantile allergic diseases could be affected by intrauterine PAH exposure, particularly in the early prenatal period and the risk was limited to the maternal GSTT1 null type.
BACKGROUND
METHODS
Flow diagram of the study design.
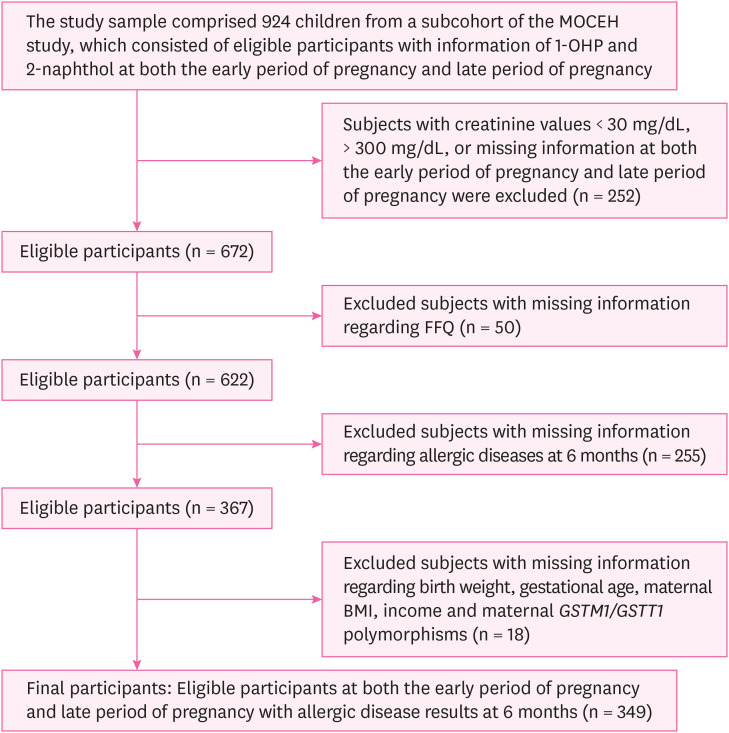
RESULTS
Comparison of general characteristics of participant 6-month-old infants by allergic diseases
Distribution of 1-OHP and 2-naphthol levels in the maternal urine with correction for creatinine concentration at the early period of pregnancy and late period of pregnancy
Association between maternal urinary PAH metabolites and allergic diseases in 6-month-old infants
Association between maternal urinary PAH metabolites and allergic diseases in 6-month-old infants in relation to maternal GST genotypes
DISCUSSION
CONCLUSION
-
Funding: This study was supported by the MOCEH (Mothers and Children's Environmental Health) project of the National Institute of Environmental Research, Republic of Korea. This work was supported by a grant from the National Institute of Environment Research (NIER), funded by the Ministry of Environment (MOE) of the Republic of Korea (NIER-2012-00-02-089).
-
Competing interests: The authors declare that they have no competing interests.
-
Author Contributions:
Conceptualization: Koh TK, Shah S, Ha E.
Data curation: Koh TK, Shah S.
Formal analysis: Koh TK, Shah S.
Methodology: Koh TK, Shah S, Ha E.
Project administration: Koh TK, Shah S, Ha E.
Software: Koh TK, Shah S.
Writing - original draft: Koh TK, Shah S.
Writing - review & editing: Koh TK, Shah S, Ha E, Park H, Hong YC, Ha M, Kim Y, Lee BE.
Abbreviations
BMI
CI
Cr
DEP
ETS
FFQ
GM
GSD
GSH
GST
GSTM1
GSTT1
IFN
IgE
IL
ISAAC
LOD
Min.
Max.
MOCEH
OR
PAH
PCR
RERI
1-OHP
SUPPLEMENTARY MATERIALS
Supplementary Table 1
Supplementary Table 2
- 1. Mota IA, Borrego LM. Allergic response to fungal exposure. In: Viegas C, Pinheiro AC, Sabino R, Viegas S, Brandão J, Veríssimo C, editors. Environmental Mycology in Public Health. Cambridge, MA: Academic Press; 2016, 35–43.
- 2. Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol 2013;24(5):476–486. 23773154.ArticlePubMedPMC
- 3. Adnan C. Epidemiology of allergic diseases. In: Robyn EO, Stephen TH, Aziz S, editors. Middleton's Allergy Essentials. Amsterdam: Elsevier; 2017, 57–60.
- 4. Kim BK, Kim JY, Kang MK, Yang MS, Park HW, Min KU, et al. Allergies are still on the rise? A 6-year nationwide population-based study in Korea. Allergol Int 2016;65(2):186–191. 26666496.ArticlePubMed
- 5. Lee E, Lee SY, Yang HJ, Hong SJ. Epidemiology of allergic diseases in Korean children. Allergy Asthma Respir Dis 2018;6(Suppl 1):S9–S20.ArticlePDF
- 6. Yoon SJ. A Study on Research Methodology and Long-term Planning Regarding Estimation of Economic Burden of Major Diseases in Korea. Seoul: Korea University; 2009.
- 7. Gluckman P, Buklijas T, Hanson M. The Epigenome and Developmental Origins of Health and Disease. 1st ed. Cambridge, MA: Academic Press; 2016.
- 8. Murrison LB, Brandt EB, Myers JB, Hershey GK. Environmental exposures and mechanisms in allergy and asthma development. J Clin Invest 2019;129(4):1504–1515. 30741719.ArticlePubMedPMC
- 9. Menichini E, Bocca B. Polycyclic aromatic hydrocarbons. In: Caballero B, editor. Encyclopedia of Food Sciences and Nutrition. Cambridge, MA: Academic Press; 2003, 4616–4625.
- 10. Miller RL, Garfinkel R, Horton M, Camann D, Perera FP, Whyatt RM, et al. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort. Chest 2004;126(4):1071–1078. 15486366.ArticlePubMed
- 11. Rosa MJ, Jung KH, Perzanowski MS, Kelvin EA, Darling KW, Camann DE, et al. Prenatal exposure to polycyclic aromatic hydrocarbons, environmental tobacco smoke and asthma. Respir Med 2011;105(6):869–876. 21163637.ArticlePubMedPMC
- 12. Kim BJ, Lee SY, Kim HB, Lee E, Hong SJ. Environmental changes, microbiota, and allergic diseases. Allergy Asthma Immunol Res 2014;6(5):389–400. 25228995.ArticlePubMedPMC
- 13. Wen HJ, Wang SL, Chen PC, Guo YL. Prenatal perfluorooctanoic acid exposure and glutathione s-transferase T1/M1 genotypes and their association with atopic dermatitis at 2 years of age. PLoS One 2019;14(1):e0210708. 30650146.ArticlePubMedPMC
- 14. Oakley A. Glutathione transferases: a structural perspective. Drug Metab Rev 2011;43(2):138–151. 21428697.ArticlePubMed
- 15. Idowu AT, Mujeeb SO. Polymorphic human glutathione s-transferase genes may predict susceptibility to type 2 diabetes mellitus: a minireview. Int J Biomed Res 2015;6(3):139–143.ArticlePDF
- 16. Leaver MJ, George SG. A piscine glutathione S-transferase which efficiently conjugates the end-products of lipid peroxidation. Mar Environ Res 1998;46(1-5):71–74.Article
- 17. Strange RC, Spiteri MA, Ramachandran S, Fryer AA. Glutathione-S-transferase family of enzymes. Mutat Res 2001;482(1-2):21–26. 11535245.ArticlePubMed
- 18. Breton CV, Vora H, Salam MT, Islam T, Wenten M, Gauderman WJ, et al. Variation in the GST mu locus and tobacco smoke exposure as determinants of childhood lung function. Am J Respir Crit Care Med 2009;179(7):601–607. 19151192.ArticlePubMedPMC
- 19. Kabesch M, Hoefler C, Carr D, Leupold W, Weiland SK, von Mutius E. Glutathione S transferase deficiency and passive smoking increase childhood asthma. Thorax 2004;59(7):569–573. 15223862.ArticlePubMedPMC
- 20. Pemble S, Schroeder KR, Spencer SR, Meyer DJ, Hallier E, Bolt HM, et al. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem J 1994;300(Pt 1):271–276. 8198545.ArticlePubMedPMCPDF
- 21. Brockmöller J, Gross D, Kerb R, Drakoulis N, Roots I. Correlation between trans-stilbene oxide-glutathione conjugation activity and the deletion mutation in the glutathione S-transferase class mu gene detected by polymerase chain reaction. Biochem Pharmacol 1992;43(3):647–650. 1540219.ArticlePubMed
- 22. Langkamp DL, Lehman A, Lemeshow S. Techniques for handling missing data in secondary analyses of large surveys. Acad Pediatr 2010;10(3):205–210. 20338836.ArticlePubMedPMC
- 23. Kim H, Cho SH, Kang JW, Kim YD, Nan HM, Lee CH, et al. Urinary 1-hydroxypyrene and 2-naphthol concentrations in male Koreans. Int Arch Occup Environ Health 2001;74(1):59–62. 11196083.ArticlePubMedPDF
- 24. Bae S, Pan XC, Kim SY, Park K, Kim YH, Kim H, et al. Exposures to particulate matter and polycyclic aromatic hydrocarbons and oxidative stress in schoolchildren. Environ Health Perspect 2010;118(4):579–583. 20368125.ArticlePubMed
- 25. Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 1990;5(1):46–51.Article
- 26. Okada E, Sasaki S, Kashino I, Matsuura H, Miyashita C, Kobayashi S, et al. Prenatal exposure to perfluoroalkyl acids and allergic diseases in early childhood. Environ Int 2014;65:127–134. 24486970.ArticlePubMed
- 27. Kim DW, Song S, Lee JE, Oh K, Shim J, Kweon S, et al. Reproducibility and validity of an FFQ developed for the Korea National Health and Nutrition Examination Survey (KNHANES). Public Health Nutr 2015;18(8):1369–1377. 25167205.ArticlePubMedPMC
- 28. Knol MJ, van der Tweel I, Grobbee DE, Numans ME, Geerlings MI. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int J Epidemiol 2007;36(5):1111–1118. 17726040.ArticlePubMed
- 29. Devouassoux G, Saxon A, Metcalfe DD, Prussin C, Colomb MG, Brambilla C, et al. Chemical constituents of diesel exhaust particles induce IL-4 production and histamine release by human basophils. J Allergy Clin Immunol 2002;109(5):847–853. 11994710.ArticlePubMed
- 30. Salvi SS, Nordenhall C, Blomberg A, Rudell B, Pourazar J, Kelly FJ, et al. Acute exposure to diesel exhaust increases IL-8 and GRO-α production in healthy human airways. Am J Respir Crit Care Med 2000;161(2 Pt 1):550–557. 10673199.ArticlePubMed
- 31. Bömmel H, Li-Weber M, Serfling E, Duschl A. The environmental pollutant pyrene induces the production of IL-4. J Allergy Clin Immunol 2000;105(4):796–802. 10756232.ArticlePubMed
- 32. Takenaka H, Zhang K, Diaz-Sanchez D, Tsien A, Saxon A. Enhanced human IgE production results from exposure to the aromatic hydrocarbons from diesel exhaust: direct effects on B-cell IgE production. J Allergy Clin Immunol 1995;95(1 Pt 1):103–115. 7529782.ArticlePubMed
- 33. van Grevenynghe J, Rion S, Le Ferrec E, Le Vee M, Amiot L, Fauchet R, et al. Polycyclic aromatic hydrocarbons inhibit differentiation of human monocytes into macrophages. J Immunol 2003;170(5):2374–2381. 12594260.ArticlePubMedPDF
- 34. Jedrychowski W, Galas A, Pac A, Flak E, Camman D, Rauh V, et al. Prenatal ambient air exposure to polycyclic aromatic hydrocarbons and the occurrence of respiratory symptoms over the first year of life. Eur J Epidemiol 2005;20(9):775–782. 16170661.ArticlePubMedPDF
- 35. Jerzynska J, Podlecka D, Polanska K, Hanke W, Stelmach I, Stelmach W. Prenatal and postnatal exposure to polycyclic aromatic hydrocarbons and allergy symptoms in city children. Allergol Immunopathol (Madr) 2017;45(1):18–24. 27789067.ArticlePubMed
- 36. Zhang X, Li X, Jing Y, Fang X, Zhang X, Lei B, et al. Transplacental transfer of polycyclic aromatic hydrocarbons in paired samples of maternal serum, umbilical cord serum, and placenta in Shanghai, China. Environ Pollut 2017;222:267–275. 28024810.ArticlePubMed
- 37. Perera F, Tang D, Whyatt R, Lederman SA, Jedrychowski W. DNA damage from polycyclic aromatic hydrocarbons measured by benzo[a]pyrene-DNA adducts in mothers and newborns from Northern Manhattan, the World Trade Center Area, Poland, and China. Cancer Epidemiol Biomarkers Prev 2005;14(3):709–714. 15767354.ArticlePubMedPDF
- 38. Jedrychowski WA, Perera FP, Tang D, Rauh V, Majewska R, Mroz E, et al. The relationship between prenatal exposure to airborne polycyclic aromatic hydrocarbons (PAHs) and PAH-DNA adducts in cord blood. J Expo Sci Environ Epidemiol 2013;23(4):371–377. 23299301.ArticlePubMedPMCPDF
- 39. Haynes BF, Heinly CS. Early human T cell development: analysis of the human thymus at the time of initial entry of hematopoietic stem cells into the fetal thymic microenvironment. J Exp Med 1995;181(4):1445–1458. 7699329.ArticlePubMedPMCPDF
- 40. Lobach DF, Hensley LL, Ho W, Haynes BF. Human T cell antigen expression during the early stages of fetal thymic maturation. J Immunol 1985;135(3):1752–1759. 3926883.ArticlePubMedPDF
- 41. Miller DL, Hiravonen T, Gitlin D. Synthesis of IgE by the human conceptus. J Allergy Clin Immunol 1973;52(3):182–188. 4737557.ArticlePubMed
- 42. McFadden JP, Thyssen JP, Basketter DA, Puangpet P, Kimber I. T helper cell 2 immune skewing in pregnancy/early life: chemical exposure and the development of atopic disease and allergy. Br J Dermatol 2015;172(3):584–591. 25354210.ArticlePubMed
- 43. Miller RL, Ho SM. Environmental epigenetics and asthma: current concepts and call for studies. Am J Respir Crit Care Med 2008;177(6):567–573. 18187692.ArticlePubMedPMC
- 44. Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol 2002;2(12):933–944. 12461566.ArticlePubMedPDF
- 45. Jones B, Chen J. Inhibition of IFN-gamma transcription by site-specific methylation during T helper cell development. EMBO J 2006;25(11):2443–2452. 16724115.ArticlePubMedPMC
- 46. Hertz-Picciotto I, Herr CE, Yap PS, Dostál M, Shumway RH, Ashwood P, et al. Air pollution and lymphocyte phenotype proportions in cord blood. Environ Health Perspect 2005;113(10):1391–1398. 16203253.ArticlePubMedPMC
- 47. Tang WY, Levin L, Talaska G, Cheung YY, Herbstman J, Tang D, et al. Maternal exposure to polycyclic aromatic hydrocarbons and 5′-CpG methylation of interferon-γ in cord white blood cells. Environ Health Perspect 2012;120(8):1195–1200. 22562770.ArticlePubMedPMC
- 48. Kim JH, Kim KH, Woo HY, Shim JY. Maternal cytokine production during pregnancy and the development of childhood wheezing and allergic disease in offspring three years of age. J Asthma 2008;45(10):948–952. 19085588.ArticlePubMed
- 49. Chen B, Hu Y, Jin T, Lu D, Shao M, Zheng L, et al. The influence of metabolic gene polymorphisms on urinary 1-hydroxypyrene concentrations in Chinese coke oven workers. Sci Total Environ 2007;381(1-3):38–46. 17498780.ArticlePubMed
- 50. Hayes JD, Strange RC. Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology 2000;61(3):154–166. 10971201.ArticlePubMedPDF
- 51. Raijmakers MT, Bruggeman SW, Steegers EA, Peters WH. Distribution of components of the glutathione detoxification system across the human placenta after uncomplicated vaginal deliveries. Placenta 2002;23(6):490–496. 12137747.ArticlePubMed
- 52. Krauer B, Dayer P. Fetal drug metabolism and its possible clinical implications. Clin Pharmacokinet 1991;21(1):70–80. 1914342.ArticlePubMed
- 53. Lutier S, Maître A, Bonneterre V, Bicout DJ, Marques M, Persoons R, et al. Urinary elimination kinetics of 3-hydroxybenzo(a)pyrene and 1-hydroxypyrene of workers in a prebake aluminum electrode production plant: evaluation of diuresis correction methods for routine biological monitoring. Environ Res 2016;147:469–479. 26970901.ArticlePubMed
- 54. Smith PG, Day NE. The design of case-control studies: the influence of confounding and interaction effects. Int J Epidemiol 1984;13(3):356–365. 6386716.ArticlePubMed
References
Figure & Data
REFERENCES
Citations

- Infantile allergic diseases: a cohort study prenatal fish intake and mercury exposure context
Surabhi Shah, Hae Soon Kim, Yun-Chul Hong, Hyesook Park, Mina Ha, Yangho Kim, Ji Hyen Lee, Eun-Hee Ha
BMC Public Health.2024;[Epub] CrossRef - Prenatal dietary exposure to mixtures of chemicals is associated with allergy or respiratory diseases in children in the ELFE nationwide cohort
Manel Ghozal, Manik Kadawathagedara, Rosalie Delvert, Amandine Divaret-Chauveau, Chantal Raherison, Raphaëlle Varraso, Annabelle Bédard, Amélie Crépet, Véronique Sirot, Marie Aline Charles, Karine Adel-Patient, Blandine de Lauzon-Guillain
Environmental Health.2024;[Epub] CrossRef - Prenatal dietary exposure to chemicals and allergy or respiratory diseases in children in the EDEN mother–child cohort
Manel Ghozal, Manik Kadawathagedara, Rosalie Delvert, Karine Adel-Patient, Muriel Tafflet, Isabella Annesi-Maesano, Amélie Crépet, Véronique Sirot, Marie Aline Charles, Barbara Heude, Blandine de Lauzon-Guillain
Environment International.2023; 180: 108195. CrossRef - Gene-environment interactions related to maternal exposure to environmental and lifestyle-related chemicals during pregnancy and the resulting adverse fetal growth: a review
Sumitaka Kobayashi, Fumihiro Sata, Reiko Kishi
Environmental Health and Preventive Medicine.2022; 27: 24. CrossRef - Role of GSTM1 in Hypertension, CKD, and Related Diseases across the Life Span
Rebecca Levy, Thu H. Le
Kidney360.2022; 3(12): 2153. CrossRef
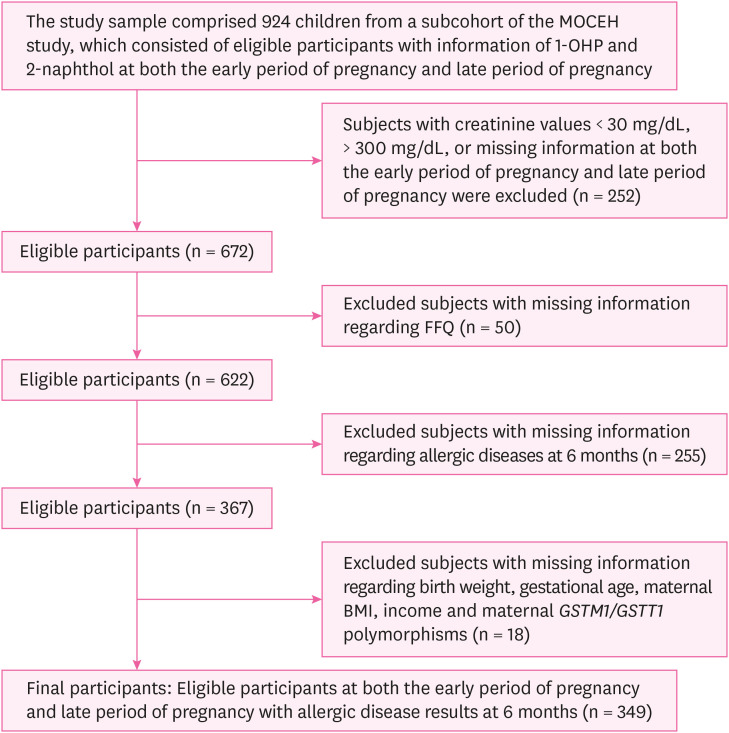
Fig. 1
| Variables | Total | Not having allergic diseases | Having allergic diseases | p-valuea | |
|---|---|---|---|---|---|
| Total | 349 (100.0) | 208 (59.6) | 141 (40.4) | < 0.01c | |
| Sex of the child | 0.86 | ||||
| Male | 191 (54.7) | 113 (54.3) | 78 (55.3) | ||
| Female | 158 (45.3) | 95 (45.7) | 63 (44.7) | ||
| Maternal age (year) | 0.06 | ||||
| < 30 | 164 (47.0) | 89 (42.8) | 75 (53.2) | ||
| ≥ 30 | 185 (53.0) | 119 (57.2) | 66 (46.8) | ||
| Maternal BMI (kg/m2) | 0.05c | ||||
| 0 < BMI <18 | 5 (1.4) | 3 (1.4) | 2 (1.4) | ||
| 18.0 ≤ BMI < 25.0 | 259 (74.2) | 145 (69.7) | 114 (80.9) | ||
| BMI ≥ 25.0 | 85 (24.4) | 60 (28.9) | 25 (17.7) | ||
| Income (million KRW/month) | 0.79 | ||||
| < 2 | 89 (25.5) | 52 (25.0) | 37 (26.2) | ||
| ≥ 2 | 260 (74.5) | 156 (75.0) | 104 (73.8) | ||
| Maternal allergic disease history | 0.01c | ||||
| No | 234 (67.1) | 150 (72.1) | 84 (59.6) | ||
| Yes | 115 (32.9) | 58 (27.9) | 57 (40.4) | ||
| Paternal allergic disease history | 0.04c | ||||
| No | 255 (73.1) | 160 (76.9) | 95 (67.4) | ||
| Yes | 94 (26.9) | 48 (23.1) | 46 (32.6) | ||
| Barbecued/fried beefb | 0.96 | ||||
| 1 time/month | 181 (51.9) | 108 (51.9) | 73 (51.8) | ||
| 2–3 times/month | 106 (30.4) | 64 (30.8) | 42 (29.8) | ||
| 1–6 times/week | 62 (17.8) | 36 (17.3) | 26 (18.4) | ||
| Barbecued/fried porkb | 0.93 | ||||
| 1 time/month | 37 (10.6) | 21 (10.1) | 16 (11.4) | ||
| 2–3 times/month | 120 (34.4) | 72 (34.6) | 48 (34.0) | ||
| 1–6 times/week | 192 (55.0) | 115 (55.3) | 77 (54.6) | ||
| Barbecued/fried chickenb | 0.35 | ||||
| 1 time/month | 122 (35.0) | 79 (38.0) | 43 (30.5) | ||
| 2–3 times/month | 138 (39.5) | 79 (38.0) | 59 (41.8) | ||
| 1–6 times/week | 89 (25.5) | 50 (24.0) | 39 (27.7) | ||
| Cotinine at early period of pregnancy (ng/mL) | 0.75 | ||||
| 0 < Cotinine < 18 | 333 (95.4) | 197 (94.7) | 136 (96.5) | ||
| 18 ≤ Cotinine < 50 | 3 (0.9) | 2 (1.0) | 1 (0.7) | ||
| Cotinine ≥ 50 | 13 (3.7) | 9 (4.3) | 4 (2.8) | ||
| Cotinine at late period of pregnancy (ng/mL) | 0.41 | ||||
| 0 < Cotinine < 18 | 334 (95.7) | 197 (94.7) | 137 (97.2) | ||
| 18 ≤ Cotinine < 50 | 5 (1.4) | 3 (1.4) | 2 (1.4) | ||
| Cotinine ≥ 50 | 10 (2.9) | 8 (3.9) | 2 (1.4) | ||
| GSTM1 | 0.57 | ||||
| Null | 192 (55.0) | 117 (56.3) | 75 (53.2) | ||
| Positive | 157 (45.0) | 91 (43.7) | 66 (46.8) | ||
| GSTT1 | 0.13 | ||||
| Null | 176 (50.4) | 98 (47.1) | 78 (55.3) | ||
| Positive | 173 (49.6) | 110 (52.9) | 63 (44.7) | ||
| PAH metabolites | Gestation (n = 349) | GM | GSD | Min | 25th percentiles | 50th percentiles | 75th percentiles | Max |
|---|---|---|---|---|---|---|---|---|
| 1-OHP (μg/g creatinine) | Earlya | 0.53 | 1.52 | 0.13 | 0.39 | 0.54 | 0.73 | 1.39 |
| Lateb | 0.54 | 1.49 | 0.16 | 0.42 | 0.55 | 0.73 | 1.94 | |
| 2-naphthol (μg/g creatinine) | Earlya | 2.12 | 1.63 | 0.28 | 1.56 | 2.21 | 2.85 | 7.35 |
| Lateb | 2.09 | 1.59 | 0.23 | 1.54 | 2.15 | 2.77 | 9.23 |
| PAH metabolites | Risk of allergic diseases in 6-month-old infants (n = 349) | ||
|---|---|---|---|
| Crude | Adjusteda | ||
| Early period of pregnancyb | |||
| 1-OHP | 1.53 (0.91, 2.58) | 1.84 (1.05, 3.22)d | |
| 2-naphthol | 1.08 (0.70, 1.68) | 1.03 (0.64, 1.65) | |
| Late period of pregnancyc | |||
| 1-OHP | 1.40 (0.81, 2.40) | 1.50 (0.83, 2.71) | |
| 2-naphthol | 0.98 (0.62, 1.56) | 0.97 (0.59, 1.61) | |
| Risk of allergic diseases in 6-month-old infantsa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | Number (%) | Early period of pregnancyb | Late period of pregnancyc | |||||||
| 1-OHP | 2-naphthol | 1-OHP | 2-naphthol | |||||||
| OR (95% CI)a | RERI (95% CI) | OR (95% CI)a | RERI (95% CI) | OR (95% CI)a | RERI (95% CI) | OR (95% CI)a | RERI (95% CI) | |||
| GSTT1 | ||||||||||
| Null | 176 (50.4) | 2.69 (1.17, 6.21)d | 2.61 (−6.02, 11.24) | 0.68 (0.33, 1.39) | −0.25 (−0.53, 0.04) | 2.37 (0.96, 5.83) | 3.62 (−7.79, 15.04) | 1.53 (0.66, 3.52) | 0.44 (−0.99, 1.88) | |
| Present | 173 (49.6) | 1.36 (0.58, 3.20) | 1.50 (0.70, 3.21) | 0.93 (0.40, 2.16) | 0.64 (0.31, 1.33) | |||||
| GSTM1 | ||||||||||
| Null | 192 (55.0) | 1.16 (0.50, 2.67) | −0.78 (−1.67, 0.11) | 0.92 (0.48, 1.76) | −0.12 (−0.52, 0.28) | 1.49 (0.65, 3.42) | 0.23 (−1.98, 2.44) | 0.97 (0.43, 2.20) | 0.01 (−0.45, 0.46) | |
| Present | 157 (45.0) | 2.23 (0.83, 5.20) | 1.31 (0.59, 2.91) | 1.35 (0.52, 3.48) | 1.09 (0.54, 2.22) | |||||
Values are presented as number (%).
BMI: body mass index;
aTested by χ2 or Fisher's exact test; bObtained from the food frequency questionnaire; cThe
PAH: polycyclic aromatic hydrocarbons; 1-OHP: 1-hydroxypyrene; GM: geometric mean; GSD: geometric standard deviation; Min.: Minimum; Max.: Maximum.
aGestational age < 20 weeks; bGestational age > 28 weeks.
Values are presented as odds ratio (95% confidence interval). Unit of the PAH metabolites, log-transformed μg/g creatinine.
PAH: polycyclic aromatic hydrocarbons; 1-OHP: 1-hydroxypyrene.
aModel adjusted for maternal age, maternal body mass index, birth weight, gestational age, infant's sex, family income, maternal allergic history, paternal allergic history, frequency of barbecued, fried, roasted or grilling beef, pork, and chicken, cotinine level (ng/mL); bGestational age < 20 weeks; cGestational age > 28 weeks; dThe
Unit of the PAH metabolites, log-transformed μg/g creatinine.
PAH: polycyclic aromatic hydrocarbons; RERI: Relative Excess Risk due to Interaction; 1-OHP: 1-hydroxypyrene; OR: odds ratio; CI: confidence interval; GST: glutathione S-transferase;
aModel adjusted for maternal age, maternal BMI, birth weight, gestational age, infant's sex, family income, maternal allergic history, paternal allergic history, frequency of barbecued, fried, roasted or grilling beef, pork, and chicken, cotinine level (ng/mL); bGestational age < 20 weeks; cGestational age > 28 weeks; dThe
 KSOEM
KSOEM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite

