The effect of blood cadmium levels on hypertension in male firefighters in a metropolitan city
Article information
Abstract
Background
This study investigated the effect of dispatch frequency on blood cadmium levels and the effect of blood cadmium levels on hypertension in male firefighters in a metropolitan city.
Methods
We conducted a retrospective longitudinal study of male firefighters who completed the regular health checkups, including a health examination survey and blood cadmium measurements. We followed them for 3 years. To investigate the effect of dispatch frequency on blood cadmium levels and the effect of blood cadmium levels on hypertension, we estimated the short-term (model 1) and long-term (model 2) effects of exposure and hypothesized a reversed causal pathway model (model 3) for sensitivity analysis. Sequential conditional mean models were fitted using generalized estimating equations, and the odds ratios (ORs) and the respective 95% confidence intervals (CIs) were calculated for hypertension for log-transformed (base 2) blood cadmium levels and quartiles.
Results
Using the lowest category of dispatch frequency as a reference, we observed that the highest category showed an increase in blood cadmium levels of 1.879 (95% CI: 0.673, 3.086) μg/dL and 0.708 (95% CI: 0.023, 1.394) μg/dL in models 2 and 3, respectively. In addition, we observed that doubling the blood cadmium level significantly increased the odds of hypertension in model 1 (OR: 1.772; 95% CI: 1.046, 3.003) and model 3 (OR: 4.288; 95% CI: 1.110, 16.554). Using the lowest quartile of blood cadmium levels as a reference, the highest quartile showed increased odds of hypertension in model 1 (OR: 2.968; 95% CI: 1.121, 7.861) and model 3 (OR: 33.468; 95% CI: 1.881, 595.500).
Conclusions
We found that dispatch frequency may affect blood cadmium levels in male firefighters, and high blood cadmium levels may influence hypertension in a dose-response manner.
BACKGROUND
Traditionally, firefighters deal with fires and rescue people from burning buildings. This role has expanded to include various duties, such as responding to accidents, disasters, emergencies, and social needs. Most firefighters work shifts and long hours to deal with unpredictable fires and other emergencies. In addition, they are exposed to high heat and various chemicals,123 including heavy metals, such as cadmium, chromium, copper, and lead,45 generated during combustion at fire extinguishing sites. For these reasons, firefighters are a high-risk occupational group for health conditions, such as cardiovascular diseases, and even sudden cardiac death.67
Hypertension is one of the most powerful modifiable risk factors for cardiovascular diseases, including coronary artery disease, peripheral vascular disease, and stroke.8910 Hypertension induces maladaptive cardiac and vascular remodeling, such as hypertrophy, interstitial fibrosis, and autonomic nervous system dysfunction, leading to cardiovascular complications.711 Hence, preventing progression to hypertension may be a pragmatic approach to lowering the risk of cardiovascular disease in firefighters.
Firefighters are exposed to multiple occupational hazards that may increase the risk of hypertension, including the inhalation of toxic heavy metals such as lead and cadmium from smoke.45 While there is considerable evidence that lead exposure can cause hypertension,1213 results of many studies are inconsistent regarding the association between mild cadmium exposure and hypertension.121415 Moreover, there have been no comprehensive studies on the relationship between dispatch frequency, blood cadmium levels, and hypertension in firefighters. We tried to address a substantial gap in the literature by investigating whether fire dispatch, considered occupational cadmium exposure, actually affects the blood cadmium levels in firefighters and increases the risk of hypertension.
To achieve the aim, we tested two hypotheses in this study: 1) the effect of dispatch frequency on blood cadmium levels, 2) the effect of blood cadmium levels on hypertension in male firefighters in a metropolitan city.
METHODS
Study design and participants
We conducted a retrospective longitudinal study among firefighters who visited a tertiary university hospital in Daegu metropolitan city in 2015 for regular health checkups. A total of 230 firefighters participated, of whom 15 were excluded as they were female. The remaining 215 male firefighters were followed up for 3 years between 2015 and 2017. No wave or item nonresponse occurred during the study period.
Measurement of dispatch frequency
The dispatch frequency was identified with the question, “How many times have you been dispatched to the fire scene last year?” Dispatch frequency was planned to be analyzed by quartiles, but the number of dispatches was found to be zero even at the 25th percentile. Hence, the analysis was performed by dividing the dispatch frequency into three groups: < 50%, 50%–75%, and ≥ 75%.
Measurement of blood cadmium levels
Venous blood samples were collected from the participants using ethylenediaminetetraacetic acid (EDTA) treated tubes. Blood samples were transferred to an analysis room and stored at 4°C. For analysis, the blood samples were thawed at room temperature and stirred, then 0.1 mL of whole blood was diluted with 0.9 mL of modifier, which included 2% hydrochloric acid, 1% ammonium dihydrogen, and 2% Triton X-100. Blood cadmium levels were measured using a non-flame atomic absorption spectrophotometer (Thermo Scientific iCE 3000 series Graphite Furnace; Thermo Fisher Scientific, Cambridge, UK). The analytical wavelength was 228.8 nm, and the injection temperature was 30°C. Zeeman calibration was used for background correction, and a standard cadmium solution (1,000 mg/L) (Sigma-Aldrich, St. Louis, MO, USA) was prepared before the measurements. The measurements were performed in 3 steps at a drying temperature of 100°C for 60 seconds and 150°C for 30 seconds, an ashing temperature of 600°C for 30 seconds, and an atomization temperature of 1,000°C for 3 seconds. The peak height of each sample was measured at the atomization temperature. The analytical laboratory passed the quality assurance program implemented by the Korea Occupational Safety and Health Agency (KOSHA) under the Industrial Safety and Health Act.
Measurement of blood pressure and definition of hypertension
Blood pressure was measured in a sitting position after resting for 5 minutes. An automated sphygmomanometer (BP-203RV III; Colin, Tokyo, Japan) was used. Participants also responded to questionnaires regarding their previous history of hypertension, including medication use. Participants with systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or taking antihypertensive agents were classified into the hypertensive group based on the criteria presented in the 7th Report of the Joint National Committee (JNC) on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.16
Covariates
Sociodemographic factors, including age, education level, smoking status, alcohol intake, exercise level, and occupational factors including work schedule, work department, and dispatch frequency, were reported via questionnaires. Educational level was classified as high school graduate or below, junior college graduate, and university graduate. Smoking status was classified as never-smoker (had smoked < 100 cigarettes in their lifetime), former smoker (had smoked ≥ 100 cigarettes in their lifetime and were not smoking at the time of the interview), and current smoker (had smoked ≥ 100 cigarettes in their lifetime and were smoking at the time of the interview). Alcohol intake was classified as never, less than once per week, once or twice per week, or three or more times per week. Exercise level was categorized as less than once per week, once or twice per week, or 3 or more times per week. The work schedule was categorized as daytime or shift work. The work department was categorized as non-administrative (including fire suppression, rescue, emergency medical service, and fire site investigation) or administrative.
Health-related factors, including body mass index (BMI) and presence/absence of diabetes mellitus, were obtained using measurement and questionnaires. BMI was calculated as weight/square of height (kg/m2) and measured using an automatic body measuring machine FA600 (Fanics, Busan, Korea). Blood sampling was conducted in the morning after participants had fasted for at least 8 h. Diabetes mellitus was defined as a fasting blood glucose level of ≥ 126 mg/dL or taking medication with a previous diagnosis of diabetes.
Potential confounders were identified through a literature review. To investigate the effect of dispatch frequency on blood cadmium levels, we set variables including age,171819 education level,20 work department,21 and work schedule22 as confounding variables. In addition, to investigate the effect of blood cadmium levels on hypertension, we set age,1419232425 BMI,19232425 smoking status,1419232425 alcohol intake,19232425 exercise level,19 education level,202425 work schedule,2226 work department,2125 and diabetes mellitus1925 as confounders.
Directed acyclic graphs
To formalize and present causal structures, we utilized directed acyclic graphs (DAGs). First, we defined the notation. Let X(t), L(t), Lc(t), and Y(t) denote the exposure, confounders, common ancestors of confounders, and outcome, respectively, at time t for t = 1, 2, and 3, with temporal ordering, as shown in Figs. 1 and 2. We assumed sequential ignorability in that there were no unobserved confounders, including the time-invariant attributes of a unit and common time trends.
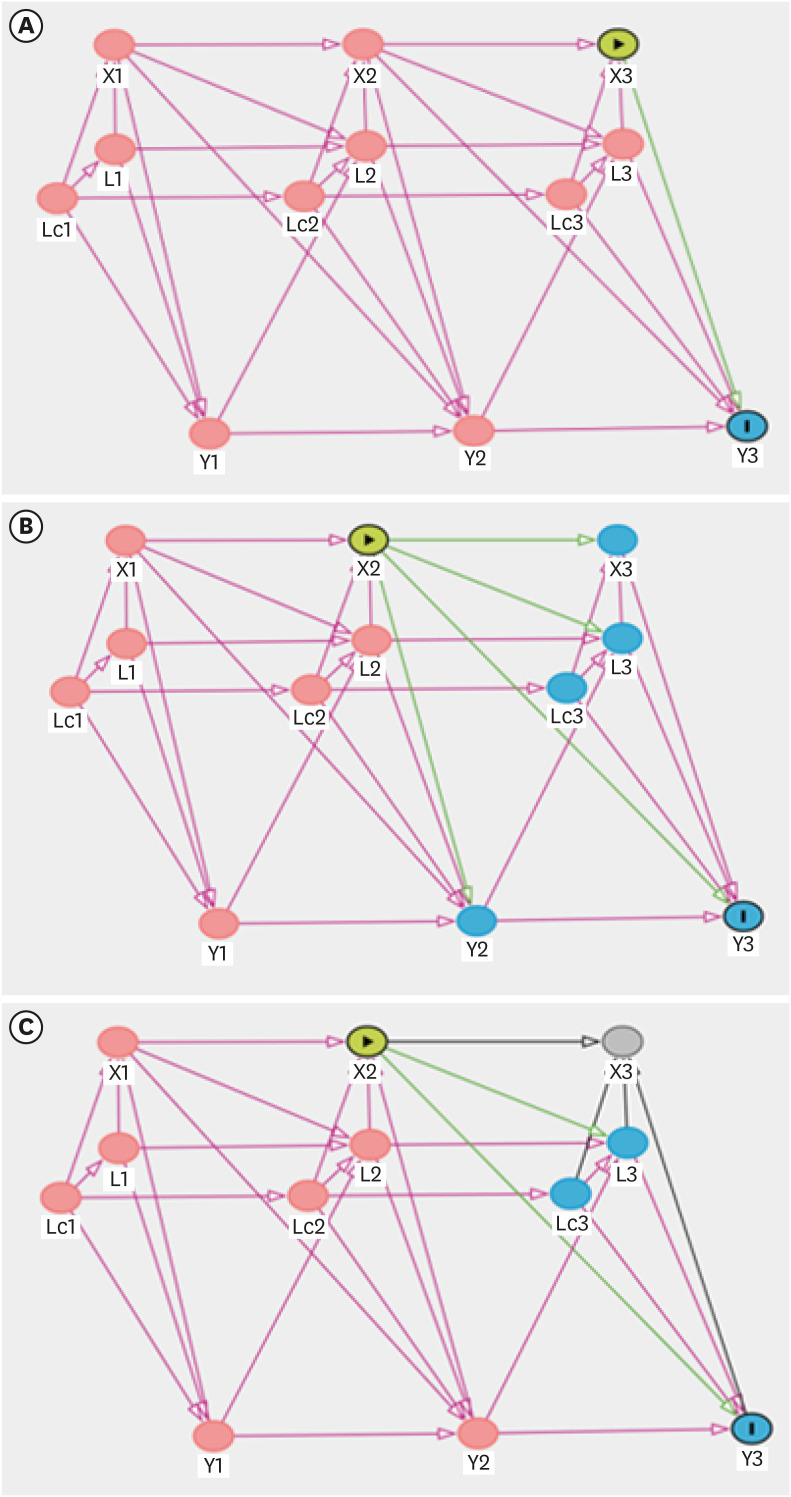
Directed acyclic graphs for three models to investigate the effect of the frequency of dispatch on blood cadmium levels. (A) Model 1 (short-term effect); (B) Model 2 (long-term effect); (C) Model 3 (reversed causal pathway model).
The green lines are the causal effect of interest, the purple lines are the backdoor paths that need to be blocked, and the black lines are neither.
X(t): the frequency of dispatch at t; L(t): work schedule and work department at t; Lc(t): age and education level at t; Y(t): blood cadmium level at t.
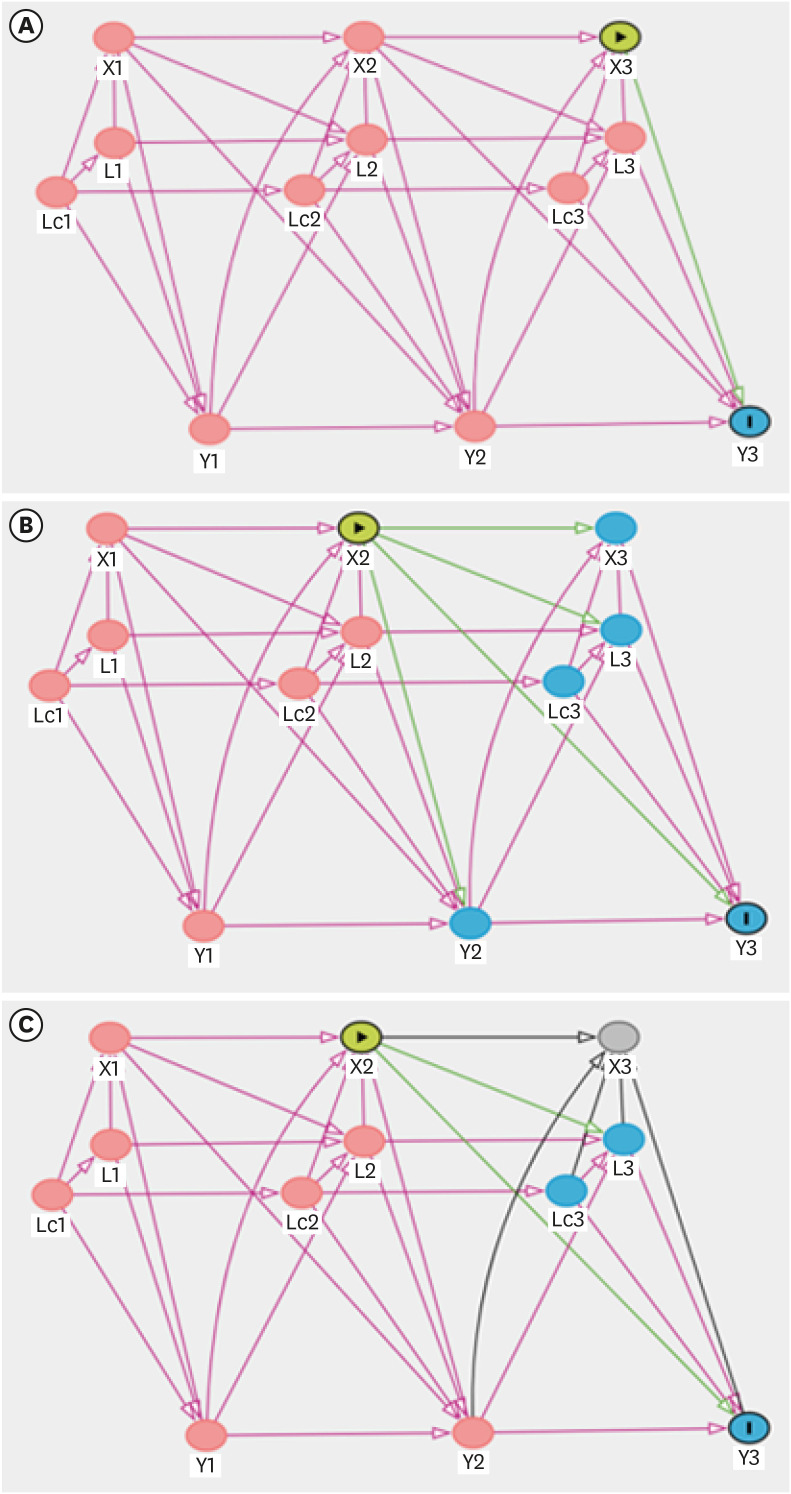
Directed acyclic graphs for three models to investigate the effect of blood cadmium levels on hypertension. (A) Model 1 (short-term effect); (B) Model 2 (long-term effect); (C) Model 3 (reversed causal pathway model).
The green lines are the causal effect of interest, the purple lines are the backdoor paths that need to be blocked, and the black lines are neither.
X(t): blood cadmium level at t; L(t): smoking, alcohol, exercise, body mass index, work schedule, work department, and diabetes mellitus at t; Lc(t): age and education level at t; Y(t): hypertension at t.
Dispatch frequency and blood cadmium levels
In Fig. 1, we illustrate the temporal ordering of the variables at time points 1, 2, and 3.
-
(1) L(t) affects X(t), L(t+1), and Y(t): it follows the definition of confounders.
• L(t) affects X(t): work schedule and work department affect dispatch frequency.
• L(t) affects Y(t): work schedule and work department affect blood cadmium levels.
• Lc(t) affects X(t), L(t), Lc(t+1), and Y(t): age and education level affect other confounders.
• Lc(t) affects X(t): age and education level affect dispatch frequency. Depending on the age and education level of firefighters, they may have different work positions, which affects dispatch frequency.
Lc(t) affects L(t): age and education level affect the work schedule and work department.
• Lc(t) affects Y(t): age and education level affect blood cadmium levels.
-
(2) Y(t) affects L(t+1) and Y(t+1)
• Y(t) affects L(t+1): recognition of blood cadmium level affects smoking, alcohol consumption, exercise level, BMI, diabetes mellitus, work schedule, and work department through lifestyle modification and post-examination health care management; recognition of blood cadmium does not directly affect the frequency of dispatch, so the effect of Y(t) on X(t+1) is excluded.
-
(3) X(t) affects X(t+1), L(t+1), Y(t), and Y(t+1)
• X(t) affects L(t+1): a person with a high frequency of dispatch may be assigned to a department with a low frequency of dispatch in the future (job rotation system).
• X(t) affects Y(t+1): the frequency of dispatch has a long-term direct effect on the blood cadmium level.
Blood cadmium levels and hypertension
In Fig. 2, we illustrate the temporal ordering of the variables at time points 1, 2, and 3.
-
(1) L(t) affects X(t), L(t+1), and Y(t): it follows the definition of confounders.
• L(t) affects X(t): smoking, alcohol consumption, exercise level, BMI, work schedule, work department, and diabetes mellitus affect blood cadmium levels.
• L(t) affects Y(t): smoking, alcohol consumption, exercise level, BMI, work schedule, work department, and diabetes mellitus affect hypertension.
• Lc(t) affects X(t), L(t), Lc(t+1), and Y(t): age and education affect other confounders.
• Lc(t) affects X(t): age and education level affect blood cadmium levels.
• Lc(t) affects L(t): age and education level affect smoking, alcohol consumption, exercise level, BMI, work schedule, work department, and diabetes mellitus.
• Lc(t) affects Y(t): age and education level affect hypertension.
-
(2) Y(t) affects X(t+1), L(t+1), and Y(t+1)
• Y(t) affects X(t+1): hypertension reduces the glomerular filtration rate, which increases blood cadmium levels.
• Y(t) affects L(t+1): diagnosis of hypertension affects smoking, alcohol consumption, exercise level, BMI, diabetes mellitus, work schedule, and work department through lifestyle modification and post-examination health care management.
-
(3) X(t) affects X(t+1), L(t+1), Y(t), and Y(t+1)
• X(t) affects L(t+1): recognition of blood cadmium level affects smoking, alcohol consumption, exercise level, BMI, diabetes mellitus, work schedule, and work department through lifestyle modification and post-examination health care management.
• X(t) affects Y(t+1): blood cadmium levels have a long-term direct effect on hypertension.
Statistical analysis
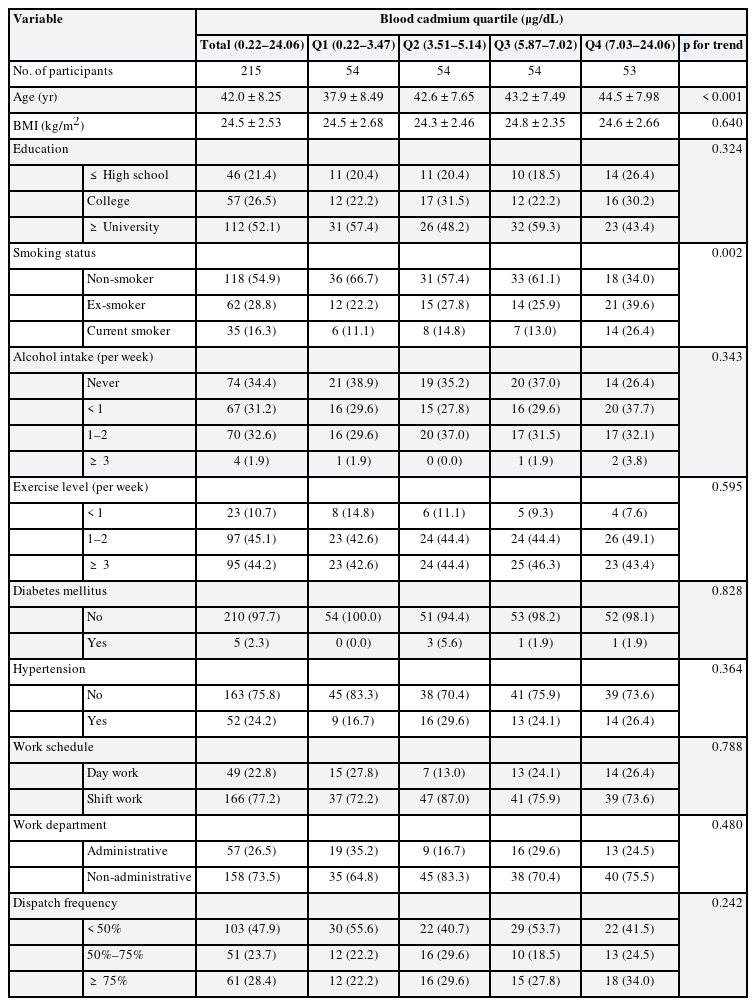
The baseline characteristics of the study participants in 2015 were presented as numbers and proportions. Participants were categorized into quartiles based on blood cadmium levels, and their sociodemographic, occupational, and health-related factors were analyzed using the Cochran-Armitage test and the linear-by-linear test.
We investigated the effect of dispatch frequency on blood cadmium levels. We also investigated the effects of blood cadmium levels on hypertension. Blood cadmium levels were presented in two different ways: 1) as a continuous log-transformed (base 2) variable and 2) as quartiles. We estimated the short-term and long-term effects of exposure in both cases and hypothesized a reversed causal pathway model for sensitivity analysis as exposure and outcome were measured simultaneously.
Sequential conditional mean models (SCMMs) were fitted using generalized estimating equations (GEE), and the odds ratios (ORs) and respective 95% confidence intervals (CIs) were calculated.
Detailed descriptions of the SCMM have been published elsewhere.2728 Briefly, SCMMs are standard regression methods that can estimate the total effect of exposure on the subsequent outcome, even in the presence of time-dependent confounding, by controlling for prior exposures, outcomes, and time-varying covariates. SCMMs enable more precise inferences with greater robustness against model misspecification and can easily accommodate continuous exposures and interactions.
DAGitty version 3.0, a web application, was used to formalize causal structures. Statistical analyses were performed using STATA version 16.0 (Stata Corp., College Station, TX, USA). A p-value of < 0.05 was considered statistically significant.
Ethics statement
This study was approved by the Institutional Review Board of Keimyung University Hospital (IRB No.2022-01-034).
RESULTS
Baseline general characteristics
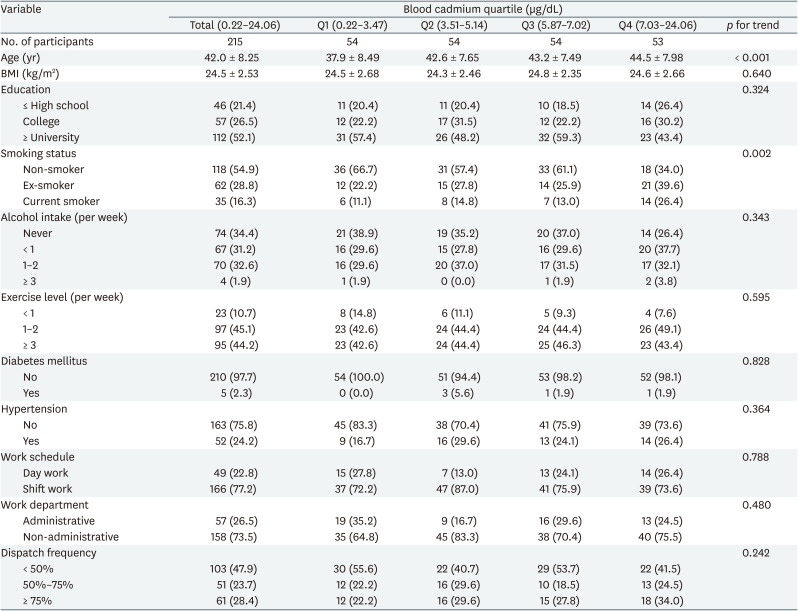
The baseline sociodemographic, occupational, and health-related characteristics of the participants are presented in Table 1. The mean age of the 215 men included in the current analysis was 42.0 years, ranging from 24 to 58 years at baseline. Of these, 54.9% were non-smokers, 28.8% were ex-smokers, and 16.3% were current smokers. At baseline, overall, most participants were non-hypertensive (75.8%), and the rates were highest among shift workers (77.2%), and non-administrative departments (73.5%). Blood cadmium levels ranged from 0.22 to 24.06 μg/dL with a median value of 5.14 μg/dL. Blood cadmium levels were positively associated with age (p < 0.001) and smoking status (p = 0.002). The geometric mean of blood cadmium levels by subgroups at baseline was presented in Supplementary Table 1.
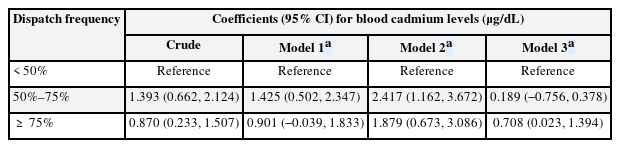
The effect of dispatch frequency on blood cadmium level
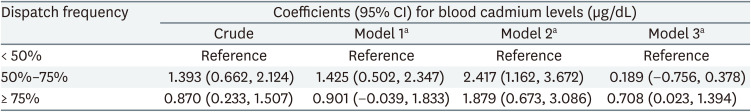
The regression coefficients for blood cadmium levels across the categories of dispatch frequency are summarized in Table 2. Using the lowest category of dispatch frequency as a reference, the highest category showed an increase in blood cadmium levels by 1.879 (95% CI: 0.673, 3.086) μg/dL and 0.708 (95% CI: 0.023, 1.394) μg/dL in models 2 and 3, respectively. In model 1, the highest category showed an increase in blood cadmium levels, but this was not statistically significant (OR: 0.901; 95% CI: −0.039, 1.833). A dose-response relationship between dispatch frequency and blood cadmium levels was not observed in models 1 and 2. GEE results assuming no dynamic relationships, including feedback and carry-over effects among variables, are presented in Supplementary Table 2.
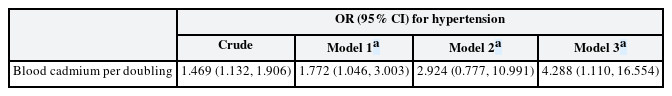
The effect of blood cadmium levels on hypertension
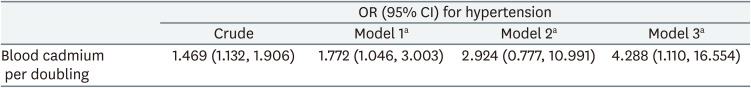
The ORs for the effect of doubling blood cadmium levels on hypertension are summarized in Table 3. Each doubling in blood cadmium level increased the odds of hypertension in models 1 and 3 (OR: 1.772; 95% CI: 1.046, 3.003 and OR: 4.288; 95% CI: 1.110, 16.554, respectively). In model 2, doubling the blood cadmium level increased the odds of hypertension but not significantly (OR: 2.924; 95% CI: 0.777, 10.991). The predictive margins of blood cadmium levels on hypertension while keeping the rest of the covariates constant are presented in Supplementary Fig. 1. GEE results assuming no dynamic relationships, including feedback and carry-over effects among variables, are presented in Supplementary Table 3.
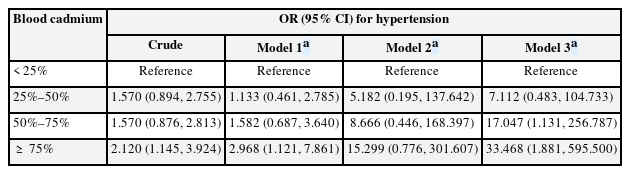
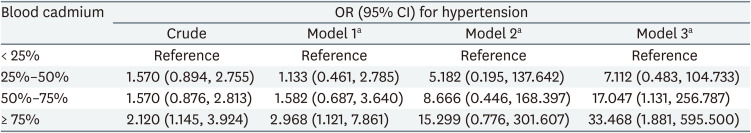
The ORs for hypertension across the quartiles of blood cadmium levels are summarized in Table 4. Using the lowest quartile of blood cadmium levels as a reference, the highest quartile showed increased odds of hypertension in models 1 and 3 (OR: 2.968; 95% CI: 1.121, 7.861 and OR: 33.468; 95% CI: 1.881, 595.500, respectively). In model 2, the highest quartile showed increased odds of hypertension, but this was not statistically significant (OR: 15.299; 95% CI: 0.776, 301.607). A dose-response relationship between blood cadmium levels and hypertension odds was observed in all models. GEE results assuming no dynamic relationships, including feedback and carry-over effects among variables, are presented in Supplementary Table 4.
DISCUSSION
This study aimed to investigate the effect of dispatch frequency on blood cadmium levels and the effect of blood cadmium levels on hypertension in male firefighters in a metropolitan city. We found that dispatch frequency may affect blood cadmium levels in male firefighters, and high blood cadmium levels, even in the normal range, may influence hypertension in a dose-response manner.
Several potential mechanisms have been proposed to explain the effects of cadmium exposure on hypertension. These include: 1) the depletion of endogenous antioxidants, 2) renal tubular epithelial cell injury, and 3) endothelial dysfunction. The cadmium–metallothionein complex, mainly formed in the liver and transported to the kidney, passes through the glomerulus, decomposes after reabsorption, and releases cadmium ions that cause oxidative stress.2930 In tubular epithelial cells, the sodium-potassium pump (Na+/K+-ATPase) can be exposed to oxidative stress, resulting in decreased sodium transport activity.31 Consequently, it leads to sodium and water retention, and prolonged volume overload alters the pressure natriuresis response, which may cause an initial increase in blood pressure to be sustained.3233 Cadmium may also facilitate the inflammatory process in atherosclerosis by releasing proinflammatory mediators from the endothelium.34 Other potential mechanisms include activation of the autonomic nervous system via calcium channels,35 directly reduced coronary flow,36 increased activity of the renin-angiotensin system,37 and increased peripheral resistance due to arteriolar contraction.38
This study is the first to examine whether cadmium exposure affects hypertension in male firefighters. Furthermore, our study adds to the accumulating evidence that cadmium exposure is associated with hypertension, as demonstrated in populations in the United States,25394041 Canada,14 China,42 and Korea.12192443 Our findings are consistent with the analysis of the US National Health and Nutrition Examination Survey’s (NHANES) 1999-2014 data that showed an association between blood cadmium levels and blood pressure.41 A Korean study using NHANES 2008-2013 data also found an association between blood cadmium levels and blood pressure.12
However, several studies have found inverse21234445 or no association between cadmium exposure and hypertension.464748 These inconsistent findings might stem from differences in the study population and methodological approaches, such as the study designs (e.g., cross-sectional or longitudinal), sample sizes, exposure levels, and adjustment for potential confounders. For example, a Japanese study23 showed an inverse association between cadmium exposure and blood pressure. Unlike this study, they included women but did not adjust sex, and the age of participants was limited to ≥ 50 years.
In addition, inconsistent results might be attributed to the type of biomarker used in the study (e.g., blood and urine). Several studies have reported that blood cadmium levels are positively associated with blood pressure, however, there is a lack of or an inverse association between urine cadmium and blood pressure.14244950 For example, the most recently published systematic review,50 which included studies published between 2010 and 2020, reported positive associations between blood cadmium levels and hypertension in various geographic, ethnic, and socioeconomic settings. Nevertheless, the association between urinary cadmium levels and hypertension remained unclear. The authors suggested the following possible reasons for this discrepancy: 1) the lack of consideration of renal function as an effect modifier; 2) the characteristics of blood cadmium that reflect biologically active cadmium levels more than urinary cadmium; and 3) the inconsistencies observed in the hypertension classification criteria.
According to our results, high blood cadmium levels showed a significant increase in the odds of hypertension in some models, but not in all models. We believe the reason for the above results is as follows. As the SCMMs include lagged covariates, the number of observations used to estimate the long-term effect is reduced by half as those used to estimate the short-term effect. As a result, it is thought that the model became heavier, and the confidence interval was widened. Since it is essential to minimize an omitted variable bias in terms of the causal inference, we thought it more appropriate to present the above results rather than the results of excluding confounders to make the model lighter.
We also found that the highest category of dispatch frequency showed an increase in blood cadmium levels, but the dose-response relationship was not observed in models 1 and 2. We considered 2 possible explanations for the lack of a dose-response relationship. The first is the potential for recall bias due to the inherent defect in the question of dispatch frequency. As mentioned above, the dispatch frequency was identified with the question, “How many times have you been dispatched to the fire scene last year?” Recalling past events over one year is too difficult for a subject to remember the events or allocate them to the correct period. In future studies, it will be necessary to collect more detailed and objective data on dispatch frequency. The second possibility is that the true model is a reversed causal pathway (model 3), which showed a dose-response relationship in contrast to the others.
It should also be noted that even a slight increase in the odds of hypertension or blood cadmium levels may have substantial cumulative effects, given that the results of this study were short-term and 1-year total long-term effects.
This study has several limitations. First, we conducted the analysis only for male firefighters in a metropolitan city, which may limit the generalizability of the study findings. Second, we did not consider the threshold of toxicological concern of cadmium for blood pressure. Third, recall bias was inherent in this study, as we used self-reported questionnaires. Fourth, we cannot rule out the possibility that residual confounding by other lifestyle and physiological factors, including a family history of hypertension, may have affected the results.
Nevertheless, this study has several strengths. First, several previous studies were cross-sectional, reporting only an association between cadmium exposure and hypertension. In contrast, this was a 3-year retrospective longitudinal study that provided insights into the causal mechanisms and processes of cadmium exposure in hypertension. Second, to the best of our knowledge, this study was the first to provide evidence that cadmium exposure affects hypertension in firefighters, a high-risk occupational group for cardiovascular disease. Third, we considered dynamic relationships, including feedback and carry-over effects, among the variables to reflect real-world complexity.
The findings of this study suggest the need to reduce cadmium exposure in firefighters. A reasonable approach to tackle this issue could be the following: 1) to implement preliminary exposure reduction procedures, which include dry or wet mitigation, immediately after exiting the emergency scene; 2) to transport personal protective equipment (PPE) in isolation from firefighters after the termination of an incident to reduce cross-contamination; and 3) to provide education on guidelines for PPE and lifestyle modification, which might reduce the burden of cadmium exposure.
CONCLUSIONS
We found that dispatch frequency may affect blood cadmium levels in male firefighters in a metropolitan city, and high blood cadmium levels, even within the normal range, may have an effect on hypertension in a dose-response manner. However, given the limitations of this study, further studies need to be conducted using various biomarkers of cadmium in diverse study populations. In addition, because exposures usually involve a mixture of heavy metals rather than a single one, future studies should assess the interactions among heavy metals.
Notes
Competing interests: The authors declare that they have no competing interests.
Author Contributions:
Conceptualization: Jeon YE, Chung I, Ha JC.
Data curation: Jeon YE, Kim MJ, Ha JC.
Investigation: Jeon YE, Kim MJ, Ha JC.
Supervision: Ha JC.
Writing - original draft: Jeon YE, Ha JC.
Writing - review & editing: Jeon YE, Ha JC.
Abbreviations
BMI
body mass index
Cd
cadmium
CI
confidence interval
DAG
directed acyclic graph
EDTA
ethylenediaminetetraacetic acid
GEE
generalized estimating equations
JNC
Joint National Committee
KOSHA
Korea Occupational Safety and Health Agency
NHANES
National Health and Nutrition Examination Survey
OR
odds ratio
PPE
personal protective equipment
SCMM
sequential conditional mean model
References
SUPPLEMENTARY MATERIALS
Supplementary Table 1
The geometric mean of blood cadmium levels by subgroups at baseline (2015)
Supplementary Table 2
The regression coefficients (95% CI) for blood cadmium levels across categories of dispatch frequency (assuming no dynamic relationships)
Supplementary Table 3
The ORs (95% CI) for hypertension per doubling of blood cadmium level (assuming no dynamic relationships)
Supplementary Table 4
The ORs (95% CI) for hypertension across quartiles of blood cadmium levels (assuming no dynamic relationships)
Supplementary Fig. 1
Predictive margins (solid line) with 95% CIs (shaded area) of blood cadmium levels on hypertension. (A) Model 1 (short-term effect); (B) Model 2 (long-term effect).