Articles
- Page Path
- HOME > Ann Occup Environ Med > Volume 29; 2017 > Article
- Case Report A case of generalized argyria presenting with muscle weakness
- Inha Jung1, Eun-Jeong Joo1, Byung seong Suh2, Cheol-Bae Ham1, Ji-Min Han1, You-Gyung Kim1, Joon-Sup Yeom1, Ju-Yeon Choi3, Ji-Hye Park4
-
Annals of Occupational and Environmental Medicine 2017;29:45.
DOI: https://doi.org/10.1186/s40557-017-0201-0
Published online: October 2, 2017
10000 0001 2181 989Xgrid.264381.aDepartment of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University, School of Medicine, 29 Saemunan-ro, Jongno-gu, Seoul, 03181 South Korea 
20000 0001 2181 989Xgrid.264381.aDepartment of Occupational and Environmental Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea 
30000 0001 2181 989Xgrid.264381.aDepartment of Dermatology, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, South Korea 
40000 0001 2181 989Xgrid.264381.aDepartment of Dermatology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, South Korea 
© The Author(s). 2017
Open AccessThis article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
Abstract
-
Background Argyria is a rare irreversible cutaneous pigmentation disorder caused by prolonged exposure to silver. Herein, we report a case of generalized argyria that developed after chronic ingestion of soluble silver-nano particles and presented with muscle weakness.
-
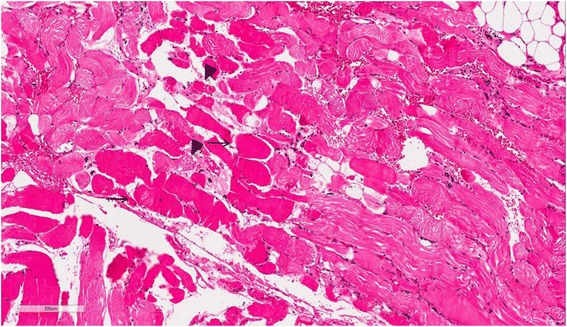
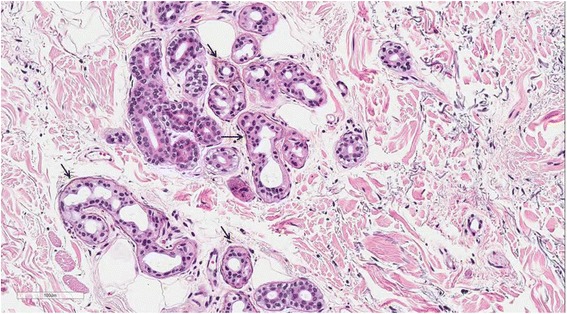
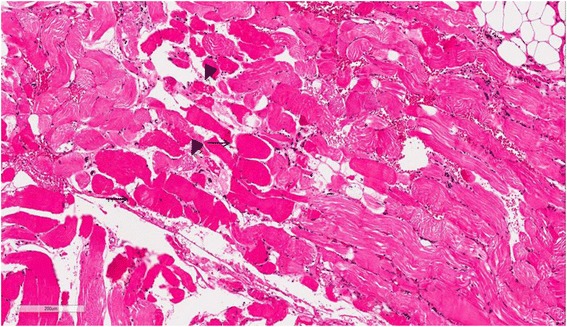
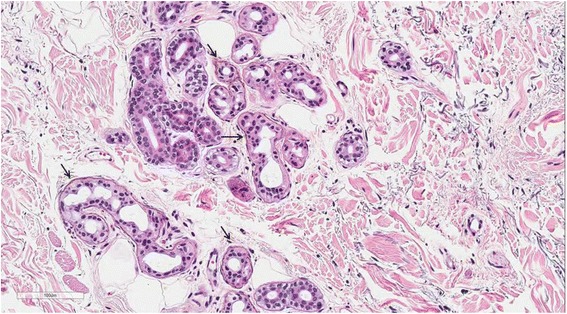
Case presentation A 74-year-old woman visited our emergency room, complaining of fever and mental deterioration. She was diagnosed with acute pyelonephritis and recovered after antibiotic therapy. At presentation, diffuse slate gray-bluish pigmented patches were noticed on her face and nails. Two months prior to visiting our hospital, she was diagnosed with inflammatory myopathy and given steroid therapy at another hospital. We performed a nerve conduction study that revealed polyneuropathy. In skin biopsies from pigmented areas of the forehead and nose, the histopathologic results showed brown-black granules in basement membranes of sweat gland epithelia, which are diagnostic findings of argyria. We reviewed pathology slides obtained from the left thigh muscles and found markedly degenerated myofibers with disorganization of myofibrils without inflammatory reactions, consistent with unspecified myopathy, rather than inflammatory myopathy. The patient was diagnosed with generalized argyria with polyneuropathy and myopathy and transferred to a rehabilitation institution after being tapered off of steroids.
-
Conclusions Clinicians should be aware of clinical manifestations of argyria and consider it in differential diagnosis when they examine patients who present with skin pigmentation and muscle weakness.
Background
Case presentation
Discussion
Conclusions
Acknowledgements
Abbreviations
- 1. Timmins AC, Morgan GA. Argyria or cyanosis. Anaesthesia 1988;43(9):755–756. 10.1111/j.1365-2044.1988.tb05748.x. 2459987.ArticlePubMed
- 2. Rongioletti F, Robert E, Buffa P, Bertagno R, Rebora A. Blue nevi-like dotted occupational argyria. J Am Acad Dermatol 1992;27(6 Pt 1):1015–1016. 10.1016/S0190-9622(08)80271-X. 1479083.ArticlePubMed
- 3. Bleehen SS, Gould DJ, Harrington CI, Durrant TE, Slater DN, Underwood JC. Occupational argyria; light and electron microscopic studies and X-ray microanalysis. Br J Dermatol 1981;104(1):19–26. 10.1111/j.1365-2133.1981.tb01706.x. 7459266.ArticlePubMed
- 4. Gulbranson SH, Hud JA, Hansen RC. Argyria following the use of dietary supplements containing colloidal silver protein. Cutis 2000;66(5):373–374. 11107524.PubMed
- 5. Furchner JE, Richmond CR, Drake GA. Comparative metabolism of radionuclides in mammals-IV. Retention of silver-110m in the mouse, rat, monkey, and dog. Health Phys 1968;15(6):505–514. 10.1097/00004032-196812000-00005. 4972147.ArticlePubMed
- 6. Shelley WB, Shelley ED, Burmeister V. Argyria: the intradermal "photograph," a manifestation of passive photosensitivity. J Am Acad Dermatol 1987;16(1 Pt 2):211–217. 10.1016/S0190-9622(87)80065-8. 3819055.ArticlePubMed
- 7. Buckley WR, Oster CF, Fassett DW. Localized argyria. II. Chemical nature of the silver containing particles. Arch Dermatol 1965;92(6):697–705. 10.1001/archderm.1965.01600180089018. 5846327.ArticlePubMed
- 8. Fung MC, Bowen DL. Silver products for medical indications: risk-benefit assessment. J Toxicol Clin Toxicol 1996;34(1):119–126. 10.3109/15563659609020246. 8632503.ArticlePubMed
- 9. Wadhera A, Fung M. Systemic argyria associated with ingestion of colloidal silver. Dermatol Online J 2005;11(1):12. 15748553.Article
- 10. Skalska J, Struzynska L. Toxic effects of silver nanoparticles in mammals--does a risk of neurotoxicity exist? Folia Neuropathol 2015;53(4):281–300. 10.5114/fn.2015.56543. 26785363.ArticlePubMed
- 11. Tang J, Xiong L, Wang S, et al. Influence of silver nanoparticles on neurons and blood-brain barrier via subcutaneous injection in rats. Appl Surf Sci 2008;255(2):502–504. 10.1016/j.apsusc.2008.06.058.Article
- 12. Xu F, Piett C, Farkas S, Qazzaz M, Syed NI. Silver nanoparticles (AgNPs) cause degeneration of cytoskeleton and disrupt synaptic machinery of cultured cortical neurons. Mol Brain 2013;6:29. 10.1186/1756-6606-6-29. 23782671.ArticlePubMedPMC
- 13. Payne CM, Bladin C, Colchester AC, Bland J, Lapworth R, Lane D. Argyria from excessive use of topical silver sulphadiazine. Lancet 1992;340(8811):126. 1352005.Article
- 14. Vik H, Andersen KJ, Julshamn K, Todnem K. Neuropathy caused by silver absorption from arthroplasty cement. Lancet 1985;1(8433):872. 10.1016/S0140-6736(85)92230-5. 2858727.Article
- 15. Prescott RJ, Wells S. Systemic argyria. J Clin Pathol 1994;47(6):556–557. 10.1136/jcp.47.6.556. 8063942.ArticlePubMedPMC
- 16. Sugawara N, Sugawara C. Comparative study of effect of acute administration of cadmium and silver on ceruloplasmin and metallothionein: involvement of disposition of copper, iron, and zinc. Environ Res 1984;35(2):507–515. 10.1016/0013-9351(84)90157-9. 6510399.PubMed
- 17. Tran HA, Song S. Silver toxicity masquerading as hypocaeruloplasminaemia. Pathology 2007;39(4):456–458. 10.1080/00313020701329781. 17676494.ArticlePubMed
References
Notes
Notes
Figure & Data
REFERENCES
Citations

- Blue Nail Discoloration: Literature Review and Diagnostic Algorithms
Jonathan K. Hwang, Shari R. Lipner
American Journal of Clinical Dermatology.2023; 24(3): 419. CrossRef - Systemic argyria with severe anemia (hemoglobin 2.4 g/L)
Nathan Chow, Kristen Fain, Jay Truitt, Cloyce Stetson
Baylor University Medical Center Proceedings.2022; 35(3): 382. CrossRef - Pigmentación exógena por nitrato de plata: aspectos dermatológicos y toxicológicos, a propósito de un caso
Ángela Londoño, Camila Pérez, Rodrigo Restrepo, Nathalie Morales, Miguel Martínez, Daniela Morales
Biomédica.2021; 41(2): 234. CrossRef - Clinical and Forensic Aspects of the Different Subtypes of Argyria
Luís Mota, Ricardo Jorge Dinis-Oliveira
Journal of Clinical Medicine.2021; 10(10): 2086. CrossRef - The Food Matrix and the Gastrointestinal Fluids Alter the Features of Silver Nanoparticles
Laurie Laloux, Donika Kastrati, Sébastien Cambier, Arno C. Gutleb, Yves‐Jacques Schneider
Small.2020;[Epub] CrossRef - Azure lunulae
Ramón García‐Galaviz, Braulio Martínez‐Benítez, Judith Domínguez‐Cherit
International Journal of Dermatology.2020;[Epub] CrossRef - The impact of bacterial size on their survival in the presence of cationic particles of nano-silver
Samir A. Anuj, Harsukh P. Gajera, Darshna G. Hirpara, Baljibhai A. Golakiya
Journal of Trace Elements in Medicine and Biology.2020; 61: 126517. CrossRef - Bacterial membrane destabilization with cationic particles of nano-silver to combat efflux-mediated antibiotic resistance in Gram-negative bacteria
Samir A. Anuj, Harsukh P. Gajera, Darshna G. Hirpara, Baljibhai A. Golakiya
Life Sciences.2019; 230: 178. CrossRef - N-Acetylcysteine reverses silver nanoparticle intoxication in rats
Monique Culturato Padilha Mendonça, Luiz Bandeira Ferreira, Cintia Rizoli, Ângela Giovana Batista, Mário Roberto Maróstica Júnior, Emanueli do Nascimento da Silva, Solange Cadore, Nelson Durán, Maria Alice da Cruz-Höfling, Marcelo Bispo de Jesus
Nanotoxicology.2019; 13(3): 326. CrossRef - Peripheral neuropathy associated with silver toxicity
Elie Naddaf, Peter J. Dyck, Paul J. Jannetto, David L. Murray, P. James B. Dyck
Neurology.2019; 92(10): 481. CrossRef
 KSOEM
KSOEM

 PubReader
PubReader ePub Link
ePub Link Cite
Cite

