Association between shift work and inflammatory markers in workers at an electronics manufacturing company
Article information
Abstract
Background
Shift work is known to be associated with cardiovascular disease (CVD). It has been found that inflammatory reactions are involved in the onset and progression of CVD. Therefore, the purpose of this study was to investigate the association between shift work and inflammatory markers.
Methods
Among workers at an electronics manufacturing company, 2,329 workers who had a health checkup from January 2019 to December 2019 were targeted. The general and biochemical characteristics of daytime workers and shift workers were compared through the Independent-test and the χ2 test. Through multiple linear regression analysis, the association with shift work and inflammatory markers was investigated. Through multiple logistic regression analysis, the association with shift work and high inflammatory markers
Results
The mean total leukocytes, neutrophils, monocytes, lymphocytes of shift workers were significantly higher than those of daytime worker. The mean high-sensitivity C-reactive protein (hs-CRP) of shift workers was also higher than that of daytime workers but not significantly. In multiple linear regression, shift work was associated with increase of total leukocyte count (β = 0.367, p < 0.001) and hs-CRP (β = 0.140, p = 0.005) after adjusting for all variables. In multiple logistic regression analysis, shift work showed 2.27 times risk of high leukocyte count and 1.8 times risk of high hs-CRP level compared to daytime work after adjusting for all variables.
Conclusions
This study confirmed that shift work is associated with high inflammatory markers. Considering that high inflammatory markers is independent indicator of CVD, the association between shift work and high inflammatory markers may help to understand the CVD risk of shift workers.
BACKGROUND
The International Labor Organization defines shift work as “A method of organization of working time in which workers succeed one another at the workplace so that the establishment can operate longer than the hours of work of individual workers”.1 However, shift work is generally defined as any type of work other than regular work hours, including night shifts and rotational shifts.2 According to the National Institute of Occupational Safety and Health (NIOSH), shift work is defined as any type of work other than regular work hours (7 a.m. to 6 p.m.).3
Modern society needs a 24-hour economic system with industrial development and a large number of people working at night to meet the economic and social needs.4 In the case of Korea, according to the results of the 6th Working Environment Survey conducted by the Korea Institute for Occupational Safety and Health in 2021, 9% of all workers and 11% of wage workers were shift workers.5
Shift work causes a number of health problems. Shift work is known to interfere with circadian biorhythm by allowing workers to be exposed to more light at night and preventing melatonin production.67 This poor-quality sleep and short sleep duration promoting an imbalance in appetite hormones that increase feelings of hunger and metabolic changes leading to obesity, insulin resistance, and reduced lipid tolerance.8 Disruption of circadian rhythms can have adverse consequences including the promotion or exacerbation of a wide variety of gastrointestinal disorders and diseases.9 Shift workers also commonly report psychological complaints including bad mood, depression, irritability, anxiety.10 In 2019, International Agency for Research on Cancer (IARC) concluded that “night shift work” is probably carcinogenic to humans (a Group 2A carcinogen).11
Shift work is also known to be associated with the risk of cardiovascular disease (CVD). Many studies have investigated the relationship between shift work and CVD. Torquati et al.12 showed that shift workers have higher risk of CVD morbidity and mortality than non-shift workers, with a 7.1% incremental risk for every 5 years of shift work exposure after the first 5 years. Bøggild and Knutsson13 showed that the CVD risk for shift workers was increased by 40%.
Inflammatory response is known to be involved in CVD. Every step of atherogenesis, from the development of endothelial cell dysfunction to foam cell formation, plaque formation and progression, and ultimately plaque rupture stemming from architectural instability, is driven by the inflammatory response.14 In this inflammatory process, cytokines induce the proliferation of leukocyte and the production of C-reactive protein (CRP) in the liver. Many studies have shown that elevated leukocyte count and CRP level are associated with CVD risk and that elevated leukocyte count and CRP are independent indicator of CVD.15161718
If shift work is associated with inflammation, shift workers may be at risk for CVD even if they are healthy without any special disease such as metabolic disorder. Therefore, it is necessary to investigate the association between shift work and inflammatory markers. Some previous studies have shown an association between shift work and inflammatory markers.192021 These studies have some limitations such as a small number of samples or only male subjects. In addition, these studies simply compared the quantitative comparison of groups. In cross-sectional studies, simple quantitative comparisons may have some limitations in interpretation if both groups are within the clinical normal range. It is also necessary to study whether there is a difference in elevation above clinically meaningful values. Therefore, we conducted this study in response to the need for further studies on the association between shift work and inflammatory markers.
METHODS
Study participants
This study was conducted on workers at an electronics manufacturing company who had a worker health examination conducted at a university hospital located in Changwon, Gyeongsangnam-do from January 2019 to December 2019. Of the total 3,512 workers, 2,688 were men, and 824 were women. Subjects whose exact work schedule was unknown, subjects with missing data, and pregnant women were excluded from the final study. In addition, subjects with a history of hypertension, diabetes, hyperlipidemia, chronic hepatitis B, thyroid disease, gout, CVD, stroke, rheumatoid arthritis, Crohn’s disease, or acute infectious disease such as cystitis or upper respiratory infection (URI) within the last 3 months were excluded. Finally, a total of 2,329 people, 1,662 men and 667 women, were selected as the subjects of the study.
Work schedule
Daytime work is from 8 a.m. to 5 p.m., working 5 days a week, and 2 holidays. Most shift workers had 2 shifts of 3 team, with daytime work from 8 a.m. to 10 p.m. and nighttime work from 10 p.m. to 8 a.m. The shift was conducted in the form of 4 days of day work, 2 days of holiday, 4 days of night work, and 2 days of holiday. Some workers did not work regular shifts, but had irregular night shifts, and in this case, were considered as shift workers in a broad sense if they received special health checkups according to the night work defined by Korea Occupational Safety & Health Agency.
Data collection and measures
A structured self-report questionnaire was used to collect information on the general characteristics of participants. Through this, age, marital status, educational background, smoking, drinking, regular exercise, sleep quality were investigated.
Marital status was classified as ‘married’ or ‘otherwise’ (unmarried, bereavement, separation). The educational background was classified as a ‘high school graduate or below’ or ‘a college graduate or higher’. Smoking was divided into ‘current smokers’ and ‘current non-smokers.’ Drinking was classified into ‘drinkers more than once a week’ and ‘drinkers less than twice a week.’ Exercise was defined as ‘regular exercise’ and distinguished from ‘irregular exercise’ when high-intensity exercise above 6 metabolic equivalent (MET) is over 75 minutes per week or medium-intensity exercise of 3 to 5.9 METs for more than 150 minutes per week. The quality of sleep was evaluated using the Pittsburgh Sleep Quality Index (PSQI). Seven subscales of sleep quality, sleep latency, sleep duration, sleep effectiveness, sleep distinction, use of sleep media, and daytime dysfunction were evaluated with 0 to 3 points, and at least 6 points out of a total of 21 points were defined as ‘poor sleep quality.’ In the case of job stress, a Korean Occupational Stress Scale (KOSS) was used to evaluate a total of 24 items with 1 to 4 points, and if the average of the conversion scores for each item exceeds 50.1, which is the top 50%, it was considered ‘a high-risk group.’ Weight, height, and waist circumference were measured through body measurement, and the body mass index (BMI) was calculated by dividing the weight by the square of the height (m2).
Inflammatory markers
Through blood collection, high-sensitivity C-reactive protein (hs-CRP) was measured by an automatic analyzer. According to the guideline presented by the American Heart Association and the American Centers for Disease Control and Prevention, hs-CRP level of 1 mg/L or higher and lower than 3 mg/L is classified as moderate risk for coronary artery disease, and level of 3 mg/L or higher is classified as high risk for coronary artery disease.22 Therefore, hs-CRP level of 3 mg/L or higher was defined as “high hs-CRP level.”
Peripheral blood was analyzed using an automated cell counter to determine the total number of leukocytes, neutrophils, lymphocytes, monocytes, eosinophils. The upper limit for the normal range of leukocyte count varies from 8,000 to 11,000 cells/μL depending on the guidelines. Welsh et al. showed that group with a white blood cell count of 9,180 cells/μL or more has a higher risk of CVD than the comparative group (hazard ratio: 1.64, 95% confidence interval [CI]: 1.24–2.16).23 Tamakoshi et al.24 showed that the group with 9,000 to 10,000 white blood cells (cells/μL) has a higher risk of CVD mortality (relative risk [RR]: 1.79, 95% CI: 0.97–3.71) although not statistically significant. And Liu et al. showed that the group with leukocyte count of 9,000 cells/μL or more has a higher risk of CVD than the comparative group (RR: 1.71, p < 0.05).25 So we defined leukocyte count over 9,000 cells/μL as “high leukocyte count (leukocytosis)” with reference to these studies.
Statistical analysis
To compare the general characteristics and biochemical values of daytime and shift worker groups, an independent t-test was used for the continuous variables and a χ2 test was used for the categorical variables. Since hs-CRP did not follow a normal distribution, it was calculated after log-transformation and were back-transformed to return estimates to original hs-CRP.
Multiple linear regression analysis was used to confirm the quantitative association between shift work and inflammatory markers after adjusting for age, sex, BMI, smoking, drinking, exercise, marriage, education level, occupational stress. Work type, sex, smoking, drinking, exercise, marriage, education level, occupational stress were calculated as categorical variables and age and BMI were calculated as continuous variables.
Multiple logistic regression analysis was used to calculate the odds ratio (OR) of high hs-CRP level and high leukocyte count according to the type of work. Model I was adjusted for age and gender. Model II was additionally adjusted for BMI, smoking, drinking, exercise, occupational stress. Work type, sex, smoking, drinking, exercise, marriage, education level, occupational stress were calculated as categorical variables and age and BMI were calculated as continuous variables.
For statistical analysis, SPSS version 25.0 software (SPSS, Inc., Chicago, IL, USA) was used, and for statistical significance, the p-value was set to less than 0.05.
Ethics statement
This study was approved by the institutional Review Board (IRB) of Samsung Changwon Hospital, Changwon (IRB approval No. SCMC 2022-03-007).
RESULTS
General characteristics of study participants
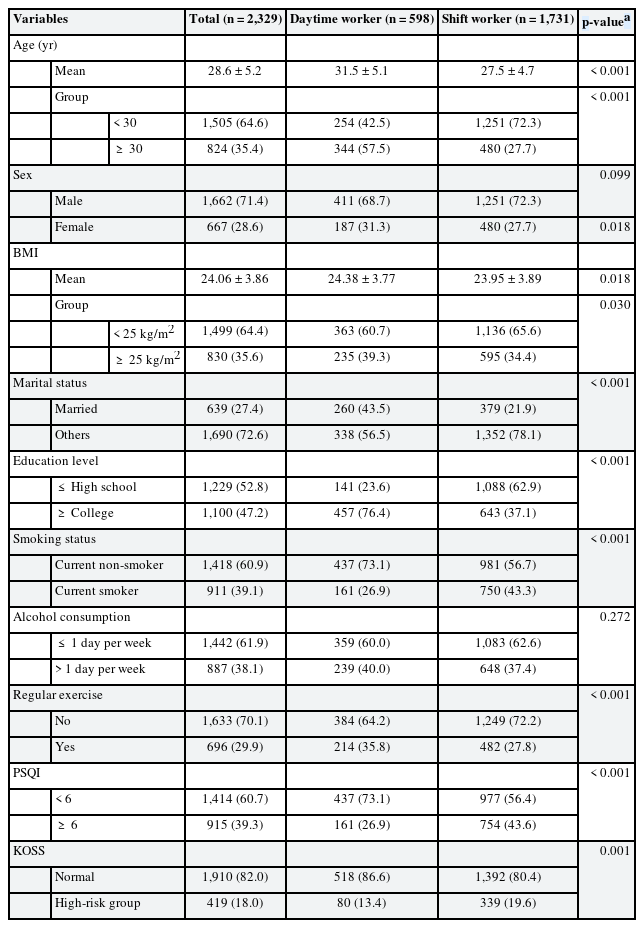
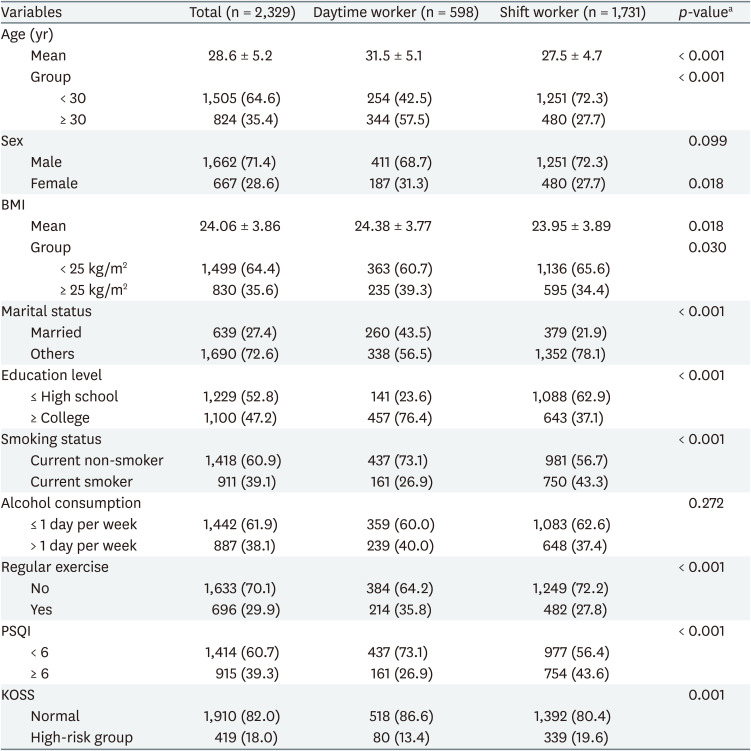
Among 2,329 subjects of the total study, 598 (25.7%) were daytime workers and 1,731 (74.3%) were shift workers. There were 1,662 (71.4%) men and 667 (28.6%) women. The average age of daytime workers was 31.5 years, which was significantly higher than the average age 27.5 years of shift worker. The proportion of workers over 30 years old was also significantly higher in daytime workers. The percentage of married people, educational background with college degree or higher, regular exercise, and obesity were significantly higher in daytime workers. Smoking, poor sleep quality, and occupational stress were significantly higher in shift workers. There was no statistically significant difference in gender and alcohol consumption between daytime workers and shift workers (Table 1).
Comparison of inflammatory markers according to work type
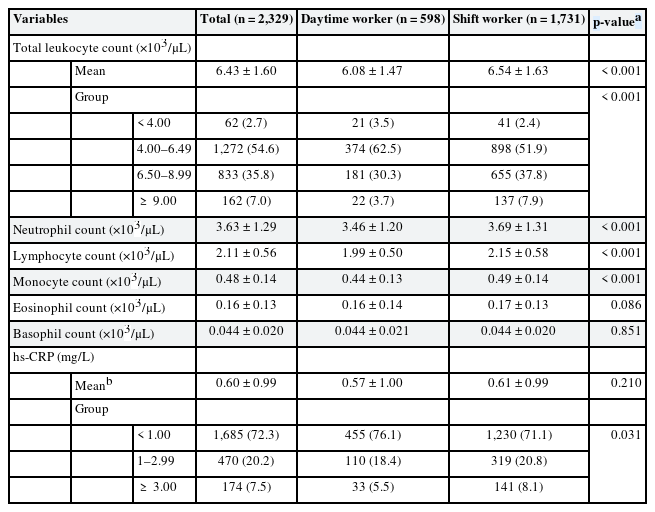
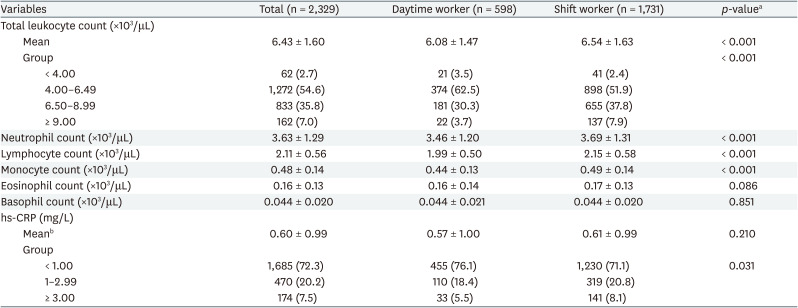
The mean of total leukocyte count was significantly higher in shift workers (6.54 ± 1.47 ×103/μL, p < 0.001) than that of daytime workers (6.08 ± 1.47 ×103/μL). When the total leukocyte count was categorized into 4 groups, there was a significant difference between daytime workers and shift workers. The proportion of people with total leukocyte count above 9.00 ×103/μL was higher in shift workers (7.9%) than in day workers (3.7%). Comparing differential leukocyte cell count results showed neutrophil, lymphocyte, and monocyte were significantly higher in shift workers. There was no statistically significant difference between eosinophil and basophil in daytime workers and shift workers.
The mean of hs-CRP was higher in shift workers (0.61 ± 0.99 mg/L, p = 0.210) than that of daytime workers (0.57 ± 1.00 mg/L), but was not statistically significant. A comparison of categorizing hs-CRP into 3 groups showed significant differences in daytime workers and shift workers, and the proportion of people with hs-CRP over 3 mg/L was higher in shift workers (8.1%) than that of daytime workers (5.5%) (Table 2).
Association of variables with total leukocyte count and hs-CRP by simple and multiple linear regression analysis
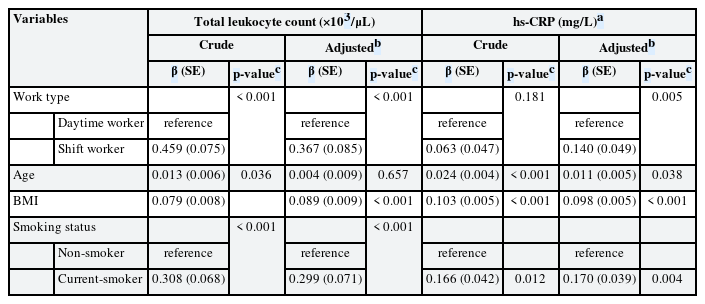
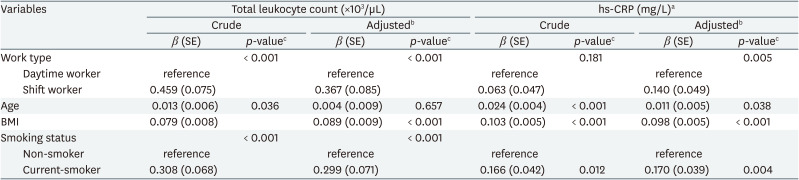
In simple linear regression analysis, shift work showed a positive significant association with total leukocyte count (β = 0.459, p <0.001), but did not show a statistically significant association with hs-CRP (β = 0.063, p = 0.181). In multiple linear regression analysis, shift work showed positive significant associations with total leukocyte count (β = 0.367, p < 0.001) and hs-CRP (β = 0.140, p = 0.005) after adjusting for age, sex, BMI, smoking status, alcohol consumption, exercise, marital status, education level, occupational stress (Table 3).
Crude and adjusted OR for high leukocyte count (leukocytosis) and high hs-CRP level according to variables
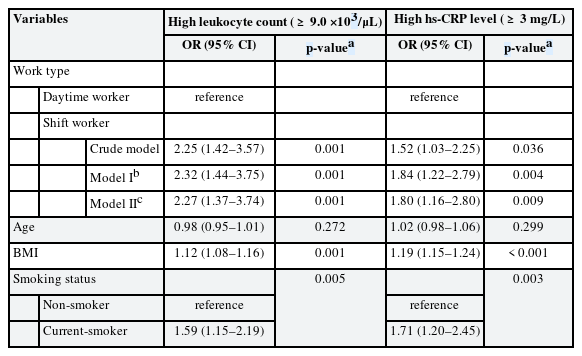
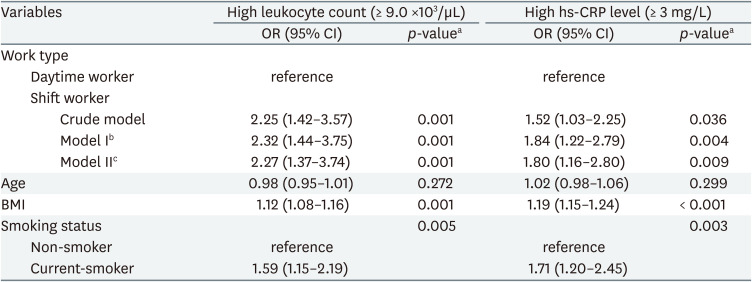
The association between shift work and high inflammatory markers was analyzed through multiple logistic regression analysis. In the crude model, the OR of high leukocyte count was 2.25 (95% CI: 1.42–3.57) in shift workers compared to daytime workers, and the OR of high hs-CRP level was 1.52 (95% CI: 1.03–2.25) in shift workers compared to daytime workers. In model I, biologically determined variables such as age, sex were corrected, and in model II, all variables were corrected. In model II, the OR of high leukocyte count was 2.27 (95% CI: 1.37–3.74) in shift workers compared to daytime workers, and the OR of high hs-CRP level was 1.80 (95% CI: 1.16–2.80) in shift workers compared to daytime workers (Table 4).
DISCUSSION
This cross-sectional study investigated the association between shift work and leukocyte count, hs-CRP as an inflammatory markers. The main finding of this study is that shift work is associated with high inflammatory markers. In the model adjusted for all covariates, the risk of “high leukocyte count” in shift workers was 2.27 times higher than that of daytime workers, and the risk of “high hs-CRP level” was 1.8 times higher than that of daytime workers.
Some previous studies have reported the association between shift work and inflammatory markers. Lu et al.26 showed that the total number of leukocytes, monocytes, neutrophils, and lymphocytes was significantly higher in steel plant shift workers than in daytime workers. Streng et al.27 showed that shift work has a positive association with total leukocyte count, monocytes, lymphocytes, and basophils compared to daytime workers in shift workers. Puttonen et al.19 showed that shift work in airline workers is associated with an increase in CRP and leukocyte count. Kim et al.21 showed a higher leukocyte count and hs-CRP in shift workers than daytime workers. These findings are consistent with our findings. We further confirmed that shift work is not only associated with elevation of inflammatory markers within the normal range, but also with elevation to clinically significant levels. In this study, there was no difference in a mean of hs-CRP between shift workers and daytime workers. However, shift work was associated with “high hs-CRP” with 3 mg/L or higher.
We compared “differential cell count” to find out whether there was a difference in the composition ratio of leukocyte subtypes by shift work and which subtype cells contributed to the elevation. In this study, neutrophils, lymphocytes, and monocytes were significantly higher in shift workers and there was no statistically significant difference between eosinophils and basophils in daytime workers and shift workers. It is very limited information, it can be considered that chronic inflammation is characterized by infiltration of monocytes and lymphocytes as the reaction progresses, starting with the initial neutrophil reaction.28 Some studies compared the absolute number of leukocyte subtypes.26293031 However, all studies, including this one, showed inconsistent results. Therefore, it seems reasonable to think that inconsistent research results suggest that inflammatory reactions caused by shift work do not involve a specific pathway or significant infiltration of a specific leukocyte subtype, but mean a complex inflammatory reaction. Much research needs to be supplemented in the future.
A number of factors are involved in the adverse health effects of shift work, including smoking, drinking, lack of exercise, and poor eating habits.32 Above all, sleep-related problems are considered the most important. Poor sleep quality, changes in sleep patterns and biological rhythms, and hormonal imbalance caused by circadian changes will be caused in shift work.33 In fact, shift workers often complain of sleep disorders due to continuous fluctuations in sleep time.34 In our study, the proportion of shift workers corresponding to the high-risk group for sleep disorders was statistically significantly higher. Shift work continuously confounds the circadian biorhythm, causing physiological stress in workers.67 This physiological stress is known to release stress hormones such as cortisol and catecholamine and induce inflammatory reactions through hypothalamic-pituitary-adrenergic axis and sympathetic nervous system activation.3536 In addition, induced fat accumulation and insulin resistance induced by stress reactions activate adipokines secreted by adipocyte to induce inflammatory reactions.37
Some other variables were also associated with inflammatory markers. Age is an independent factor associated with metabolic disorders and chronic inflammation.38 In this study, age showed a positive association with both leukocyte and hs-CRP in simple linear regression analysis. However, there was no statistically significant association with high leukocyte count and high hs-CRP level, which is thought to have not shown a difference to a clinically meaningful level because the age of the subject of this study was young and the difference was not large. There are several studies that show differences in sex hormones and periodicity as biological variables that affect the function of the immune system.39 Although it was not presented in the results, our study classified and compared men and women. However, there was no statistically significant difference in our study. Inflammation can be induced by diseases such as metabolic disorders or obesity. Previous studies have reported that metabolic overload causes stress reactions such as oxidative reactions and cell hypertrophy, which promotes cell rupture that causes inflammatory reactions.36 It is also known that leptin and pro-inflammatory cytokine secreted by adipocyte cause inflammation.40 In this study, BMI was significantly associated with inflammatory markers. Smoking is also known to induce persistent systemic inflammatory reactions and local inflammatory reactions such as bronchitis, resulting in the secretion of cytokines such as tumor necrosis factor-α and interleukin-6.41 This study also showed that smoking is a strong inflammation-inducing factor. Drinking,42 exercise,43 and occupational stress,44 which had association with inflammatory markers in previous studies, had no significant association with inflammatory markers, but were corrected in the adjusted model II along with other variables.
In general, shift work is known to be associated with CVD risk. Chronic inflammation is known to be involved in atherosclerosis and insulin resistance, the main mechanism of CVD onset, which is characterized by increase of inflammatory cytokines, leukocytes, complements, and CRP in response to physiological stressors.45 Elevated leukocyte count and elevated CRP by inflammation are known as independent indicators with significant association with CVD.15161718 Therefore, our finding that shift work is associated with high inflammatory markers that can be potential CVD risk factor can help explain the association between shift work and CVD.
This study has several limitations. First, this study is a cross-section design. There may be limitations in reasoning about the causal relationship between shift work and elevated inflammatory markers. In subsequent studies, it seems necessary to grasp the causal relationship by longitudinal studies. Second, the dates on which the subjects' tests were conducted are different. Some groups of shift workers performed examinations after holidays, some groups performed after day work, and some workers performed after night work. This limits explaining the chronic effect of shift work due to the effect of short-term effects according to the type of work. Third, all of the types of shift work in the study subjects were 2 shifts of 3 team, which means that the effect of the shift work type could not be confirmed. In fact, Streng et al.27 showed that the number of night shifts per month and the number of consecutive night shifts were associated with an increase in leukocyte count, even in the same shift. Fourth, daytime workers and shift workers showed differences in general characteristics, and there is a possibility that there is a disturbance variable that has not been investigated. In fact, daytime workers have a high proportion of office workers, and shift workers mainly work in the manufacturing process. Fifth, it is the result of a study on workers in an electronics manufacturing company, but attention is needed to generalize and interpret the influence of shift workers in all occupations based on this. In the follow-up study, it is expected that research on various occupational groups will support the interpretation of the association between shift work and inflammatory markers in all general workers.
Nevertheless, this study was conducted at a single institution targeting a single company, and controlled as much as possible for other factors that could affect the inflammatory markers. Based on the results, it is significant in that it is a study that firmly confirmed that shift work can have association with high inflammatory markers. This will supplement the previous studies with additional information and help to understand association between shift work and inflammation. Considering that high inflammatory markers is independent indicator of CVD, the association between shift work and high inflammatory markers may help to understand the CVD risk of shift workers. If a large-scale cohort study is supported in the future, it is expected that inflammatory markers will provide information necessary for monitoring the CVD risk of individual shift workers.
CONCLUSIONS
In conclusion, our study confirmed that shift work is associated with high inflammatory markers. Considering that high inflammatory markers is independent indicator of CVD, the association between shift work and high inflammatory markers may help to understand the CVD risk of shift workers.
ACKNOWLEDGEMENTS
The authors would like to thank Chung EY for technical assistance for this study.
Notes
Competing interests: The authors declare that they have no competing interests.
Author contributions:
Conceptualization: Woo SJ.
Data curation: Chae CH, Lim JW.
Formal analysis: Woo SJ, Chae CH.
Investigation: Lim JW.
Methodology: Woo SJ, Chae CH.
Software: Woo SJ, Chae CH.
Validation: Chae CH, Lim JW.
Visualization: Woo SJ.
Writing - original draft: Woo SJ.
Writing - review & editing: Chae CH, Lim JW.
Abbreviations
BMI
body mass index
CI
confidence interval
CRP
C-reactive protein
CVD
cardiovascular disease
hs-CRP
high-sensitivity C-reactive protein
IARC
International Agency for Research on Cancer
KOSS
Korean Occupational Stress Scale
MET
metabolic equivalent
NIOSH
National Institute of Occupational Safety and Health
OR
odds ratio
PSQI
Pittsburgh Sleep Quality Index
RR
relative risk
URI
upper respiratory infection