The acclimatization of Haenyeo to a cold environment and occupational characteristics evaluated by orexin and irisin levels
Article information
Abstract
Background
Haenyeo is a woman who has the job of collecting seafood in the Jeju Sea at an average temperature of 13°C–14°C. The purpose of this study was to examine the cold acclimatization and occupational characteristics of Haenyeo through biomarkers such as orexin and irisin related to heat generation in the body.
Methods
Twenty-one Haenyeo and 25 people with similar age, body type, and body mass index were selected as the control group (Control G). In the cold exposure experiment, a climate chamber was set to 5°C and both feet were immersed in a 15°C water tank for 30 minutes. Tympanic temperature (Tty) and skin temperature (Tsk) were measured, and the mean body temperature (mTb) was calculated. Blood samples were collected before and immediately after the examination. Orexin and irisin levels were analyzed.
Results
Orexin levels were elevated after cold stimulation from 12.17 ± 4.44 to 12.95 ± 4.53 ng/mL (Haenyeo group [Haenyeo G], p < 0.01) and 10.37 ± 3.84 to 11.25 ± 4.02 ng/mL (Control G, p < 0.001). Irisin levels were elevated after cold stimulation from 4.83 ± 2.28 to 5.36 ± 2.23 ng/mL (Haenyeo G, p < 0.001) and 3.73 ± 1.59 to 4.18 ± 2.04 ng/mL (Control G, p < 0.001). The difference between Haenyeo G and Control G values in orexin and irisin appears not only in pre-exposure but also in post-exposure (p < 0.05).
Conclusions
Our experimental results suggest that Haenyeo G were relatively superior in cold tolerance to Control G under cold exposure conditions. Haenyeo’s cold acclimatization is due to the basic differences in pyrogens regarding body temperature control such as orexin and irisin. This means that Haenyeo are advantageous for cold survival.
BACKGROUND
Haenyeo means “sea woman” in Korean and refers to a woman who collects seafood from the sea without oxygenation.123 Their culture was listed as the Jeju Haenyeo Culture on the UNESCO Intangible Cultural Heritage of Humanity in 2016, and the Jeju Haenyeo Culture was listed as a diving gut praying for safety and good fish to strengthen the solidarity of the Haenyeo community.
Haenyeo women are known to withstand cold water better than other divers due to winter diving practices, and previous studies found that Haenyeo began to wear new swimsuits instead of traditional cotton swimsuits in the mid-1970s, losing their cold adaptability.4 Since then, studies on the controlled response of body temperature to the cold in Haenyeo have decreased. The number of Jeju Haenyeo currently active decreased sharply from 14,143 in 1970 to 3,603 in 2020, of which 89% are in their 60s or older.5 In particular, Jeju’s elderly Haenyeo have a history of adapting to the low-oxygen and cold environment by working at an average seawater temperature of 13°C–14°C, which lasts throughout the 4 seasons, for more than 50 years starting in their midteens.3 Therefore, in terms of maintaining homeostasis according to occupational characteristics, Haenyeo have undergone physiological adaptations due to the long time spent in a cold working environment. Thus, it is valuable from the perspective of occupational environmental medicine (OEM).6 In addition, Haenyeo have an intangible historical value with environmental physiological elements of climate adaptation as they are the only unique group in the world with both traditional cotton diving suits and modernized rubber diving suits.37
Recent Haenyeo studies related to cold adaptation reported that body temperature and energy metabolism, heart rate changes, and local cold tolerance were related to cold-induced vasodilation in cold-exposed older Haenyeo.89 However, to confirm the cold adaptation of Haenyeo, research on biomarkers to analyze the mechanisms involved in regulating heat generation reactions is needed. According to a recent study, orexin and irisin are 2 representative substances that are newly emerging as substances closely related to heat production in the body (Fig. 1).101112
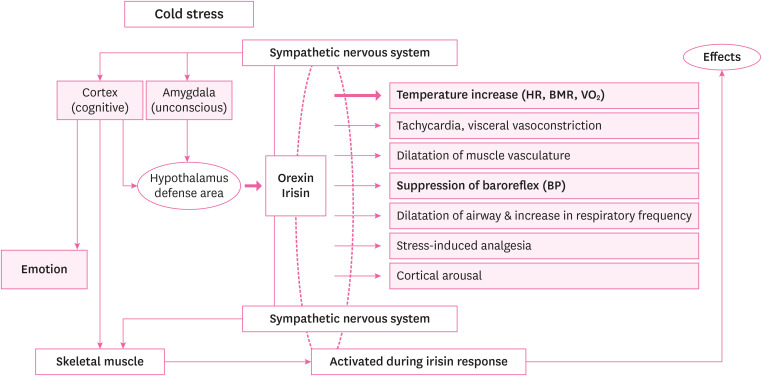
Orexin as a master switch of the orchestrated sympathetic response and thermoregulation by irisin. The sympathetic nervous system can activate the hypothalamus defense area to release orexin, and skeletal muscle to initiate the irisin response. Orexin is thought to be involved in the sympathetic pathway.
HR: heart rate; BMR: basal metabolic rate; VO2: oxygen consumption; BP: blood pressure.
Orexin is an excitatory neuropeptide hormone and has been found to be involved in sleep awakening and nutrition, as well as autonomic nervous system (ANS) function and neurohormone regulation.10131415 Studies have also shown that ANS hyperactivity and orexin systems, such as breathing and blood pressure increases, have mutual effects, with both non-shivering and shivering mechanisms, which are body temperature regulation mechanisms.15 Irisin is fibronectin type III domain-containing 5 secreted from muscles into the blood, and it increases breathing by more than 50 times in brown fat mitochondria and mitochondrial uncoupling protein 1 (UCP1), which is responsible for lipid degradation cascades.111617 However, previous studies mainly targeted cell lines or experimental animals or used partial cold exposure, so there is a limit to the generalizability of the results.1315
The purpose of this study was to examine the environmental physiological and occupational characteristics of maintaining body temperature in the cold acclimatization of Haenyeo by analyzing biomarkers such as orexin and irisin. However, systematic research was not conducted, raising the need for a more comprehensive study.
METHODS
Subjects
This study was conducted from January 2017 to December 2019, and in the experimental group (Haenyeo), the participants were recruited with the help of the Jeju Haenyeo Museum and the Jeju fishing village community. The research manager directly explained the overall outline, purpose, and academic value of the experiment at the Fishing Village and Haenyeo Museum, and recruited subjects for those with more than 50 years of Haenyeo career.
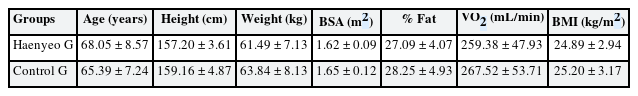
For the control group (Control G), participants with similar age, body type, and body mass index (BMI) were recruited as the selection criteria based on the basic body data of the Haenyeo. In addition, the Control G recruited those who lived in Jeju and had never worked as Haenyeo. The physical characteristics of all study participants are presented in Table 1. Body surface area was calculated using the Du Bois formula.1819 Body fat percentage was measured using the bioimpedance method (InBody 520; Biospace Inc., Seoul, Korea).19 The oxygen uptake measurement consisted of evaluating gas samples that had expired for 30 minutes after resting for 10 minutes in a lying position. Oxygen inhalation was measured using a computerized gas analyzer (Quark PFT; Cosmed, Rome, Italy).19 Each subject provided written informed consent after being thoroughly acquainted with the purpose and experimental procedures as well as any potential risks.
The following exclusion criteria were established based on the results of simple interviews and general health examination conducted in Jeju Island while selecting subjects from the Haenyeo group (Haenyeo G) and Control G. Those diagnosed with hypertension, diabetes, dyslipidemia, cardiovascular disease (ex. myocardial infarction), neurovascular disease (ex. stroke), or those under medication or current smokers were excluded from this study. In addition, people with health problems measured as diagnostic criteria for high blood pressure (2-stage hypertension with systolic blood pressure ≥ 160 mmHg or diastolic blood pressure ≥ 100 mmHg), diabetes (fasting glucose level ≥ 126 mg/dL), and dyslipidemia (at least one of total cholesterol ≥ 240 mg/dL, low-density lipoprotein-cholesterol ≥ 160 mg/dL, triglyceride ≥ 200 mg/dL and high-density lipoprotein-cholesterol < 40 mg/dL)20 in general health examinations were also excluded from this study.21
All of the participants in the study were women, and a total of 46 people were tested, including 21 Haenyeo with more than 50 years of professional experience, 25 Control G with similar age and BMI.
Measurement and experimental procedures
The test was conducted in the city of Cheonan (Chungnam), Republic of Korea. Cheonan is located in the southwest part of Korea (126°52′N, 33.38′E). It extends northeast (130°4′N, 43.0′E). The environmental conditions of the test room (climate chamber) were maintained at temperature of 5.0°C ± 0.5°C and 25.0°C ± 0.5°C; relative humidity 60.0% ± 3.0%; and air velocity 1 m/s.22 The experiment was conducted between 2 and 5 p.m. to control for the influence of circadian rhythm on body temperature. Upon arrival at the climate chamber, each subject wore light indoor clothing without shoes and socks.22 The subjects sat in a chair in a relaxed posture for 60 minutes before the start of the main process.
In the 5°C experiments, both feet were submerged in a 15°C water tank for 30 minutes. Since the cold loading experiment was conducted by setting the temperature to 5°C, the cold may be severely felt depending upon the degree of cold resistance of the subject to the experimental conditions. In these experimental conditions, no risk has been reported, but the tolerability varies from individual to individual, so if a participant wanted to stop the experiment, one researcher was always present in the laboratory in case of an emergency. In addition, as a precautionary measure, a bed blanket, an electric blanket, a hot pack, and a warm drink to maintain a warm body temperature were available, and the researchers closely monitored the participants.
Tympanic temperature (Tty) and mean body temperature (mTb) measurements
Tty was continuously assessed at 10-second intervals after 60 minutes in each experiment by inserting a TSK7+1 thermistor probe (Fig. 2) with a small spring (K923; Takara, Yokohama, Japan), which was connected to a personal computer (CF-T1; Panasonic, Tokyo, Japan) and a data logger (K-720; Technol Seven, Yokohama, Japan), into the left ear.1222 When the thermistor probe contacted the tympanic membrane, the subject felt slight discomfort and could hear a scratching noise. The inner pinna was filled with small cotton balls to fix the probe in the ear.12 The skin temperature of the chest, upper arm, thigh, and leg was measured with a TSK7+1 thermistor probe (Fig. 2) and a K923 small spring (Takara).1222 The probe was connected to a CF-T1 personal computer (Panasonic) and a K-720 data logger (Technol Seven).122223 The mean skin temperature (mTsk) was calculated using the Ramanathan equation.24 The mTb parameter was calculated using the formula of Sugenoya and Ogawa25: mTb = (0.9. Tty + 0.1 mTsk).
Blood analysis
Blood was sampled before the immersion (pre) and immediately following the examination (post). In Soonchunhyang University Physiology Laboratory 208, the researcher collected a total of 10 mL of whole blood twice before and after the experiment from each of the subjects’ median cubital veins and transferred to serum-separating tubes.12 The samples were centrifuged at 3,000 rpm (2,000 × g) for 10 minutes at 4ºC. The serum was removed and stored in 1mL aliquots at −80ºC until analysis using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Orexin A EIA Kit; Phoenix Pharmaceuticals, Burlingame, CA, USA). Irisin was determined using a commercial ELISA kit (Irisin EIA kit EK-067-52; Phoenix Pharmaceuticals).12
Statistical analysis
Descriptive statistics are expressed as the mean ± standard deviation using commercially available computer software SPSS for Windows, version 26.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was determined using a Shapiro-Wilk test to test normality and an independent t-test for comparison between Haenyeo G and Control G. In addition, a paired t-test was performed for comparison between before (pre) and after (post) cold stimulation. Significant di\\xef\\xac\\x80erences were considered at p < 0.05.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board on Human Subjects Research and Ethics Committees, Soonchunhyang University (No. 1040875-201611-BR-042). The patients and participants provided written informed consent to participate in this study.
RESULTS
Tty and mTb
As shown in Figs. 3 and 4, in the Haenyeo G, Tty decreased after the experiment (pre-post Tty in Haenyeo G, 36.70°C ± 0.14°C to 36.68°C ± 0.13°C, p < 0.001). mTb also decreased after the experiment (pre-post mTb in Haenyeo G, 36.50°C ± 0.15°C to 36.46°C ± 0.17°C, p < 0.001).
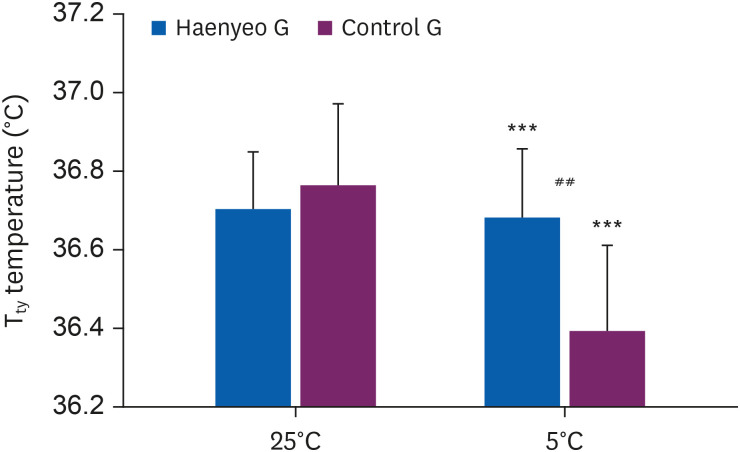
Tty (Haenyeo G vs. Control G). Comparison of Tty temperature before (pre) and after (post) cold stimulation (5°C ± 0.5°C). Values (Haenyeo G, n = 21 vs. Control G, n = 25) are presented as mean values ± standard deviation.
Tty: tympanic temperature; Haenyeo G: Haenyeo group; Control G: control group.
***p < 0.001, statistically significant difference between pre- and post-exposure values for Haenyeo G and Control G, respectively; calculated by paired t-test.
##p < 0.01, statistically difference between Haenyeo G and Control G values in post-exposure; calculated by independent t-test.
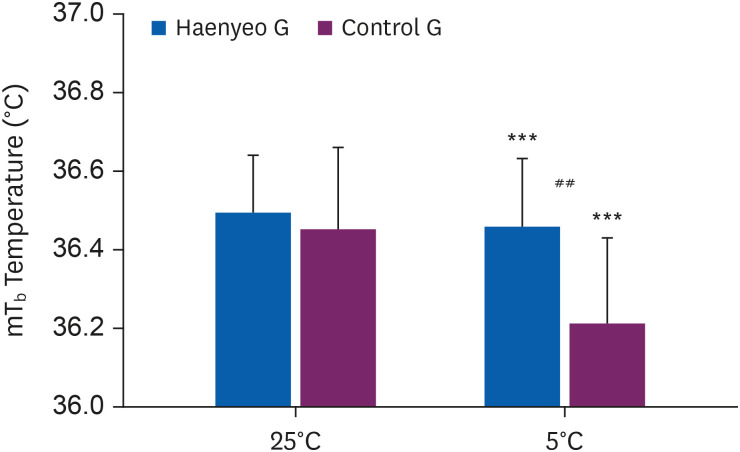
mTb (Haenyeo G vs. Control G). Comparison of mTb before (pre) and after (post) cold stimulation (5°C ± 0.5°C). Values (Haenyeo G, n = 21 vs. Control G, n = 25) are presented as mean values ± standard deviation.
mTb: mean body temperature; Haenyeo G: Haenyeo group; Control G: control group.
***p < 0.001, statistically significant difference between pre- and post-exposure values for Haenyeo G and Control G, respectively; calculated by paired t-test.
##p < 0.01, statistically significant difference between Haenyeo G and Control G values in post-exposure; calculated by independent t-test.
The Control G also decreased Tty after the experiment (pre-post Tty in control G, 36.76°C ± 0.34°C to 36.39°C ± 0.41°C, p < 0.001). mTb also decreased after the experiment (pre-post mTb in control G, 36.45°C ± 0.21°C to 36.21°C ± 0.22°C, p < 0.001).
Before the experiment, there was no difference between the Haenyeo G and the control G in both Tty (pre Tty in Haenyeo G vs. control G, 36.70°C ± 0.14°C vs. 36.76°C ± 0.34°C) and mTb (pre mTb in Haenyeo G vs. Control G, 36.50°C ± 0.15°C vs. 36.45°C ± 0.21°C).
On the other hand, after the experiment, both Tty (post Tty in Haenyeo G vs. Control G, 36.68°C ± 0.13°C vs. 36.39°C ± 0.41°C, p < 0.01) and mTb (post mTb in Haenyeo G vs. Control G, 36.46°C ± 0.17°C vs. 36.21°C ± 0.22°C, p < 0.01) between the Haenyeo G and the Control G showed statistically significant differences.
Orexin levels
As shown in Fig. 5, in the Haenyeo G, orexin increased after the experiment (pre-post orexin levels in Haenyeo G, 12.17 ± 4.44 ng/mL to 12.95 ± 4.53 ng/mL, p < 0.01). The Control G also increased orexin after the experiment (pre-post orexin levels in Control G, 10.37 ± 3.84 ng/mL to 11.25 ± 4.02 ng/mL, p < 0.001).
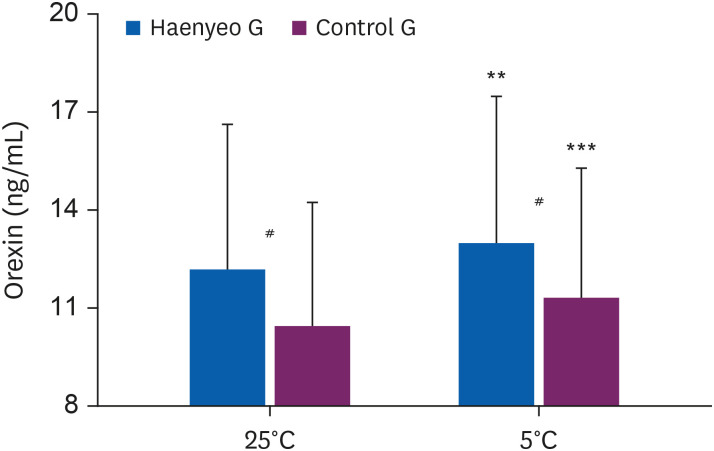
Orexin levels (Haenyeo G vs. Control G). Comparison of serum orexin levels before (pre) and after (post) cold stimulation (5°C ± 0.5°C). Values (Haenyeo G, n = 21 vs. Control G, n = 25) are presented as mean values ± standard deviation.
Haenyeo G: Haenyeo group; Control G: control group.
**p < 0.01, statistically significant difference between pre- and post-exposure values in Haenyeo G; calculated by paired t-test.
***p < 0.001, statistically significant difference between pre- and post-exposure values in Control G; calculated by paired t-test.
#p < 0.05, statistical differences in Haenyeo G and Control G values in pre-exposure, as well as post-exposure; calculated by independent t-test.
Before the experiment, orexin between the Haenyeo G and the Control G showed statistically differences (pre orexin levels in Haenyeo G vs. Control G, 12.17 ± 4.44 ng/mL vs. 10.37 ± 3.84 ng/mL, p < 0.05). Even after the experiment, there is a statistical difference in orexin between the Haenyeo G and the Control G (post orexin levels in Haenyeo G vs. Control G, 12.95 ± 4.53 ng/mL vs. 11.25 ± 4.02 ng/mL, p < 0.05).
Irisin levels
As shown in Fig. 6, in the Haenyeo G, irisin increased after the experiment (pre-post irisin levels in Haenyeo G, 4.83 ± 2.28 ng/mL to 5.36 ± 2.23 ng/mL, p < 0.001). The Control G also increased irisin after the experiment (pre-post irisin levels in Control G, 3.73 ± 1.59 ng/mL to 4.18 ± 2.04 ng/mL, p < 0.001).
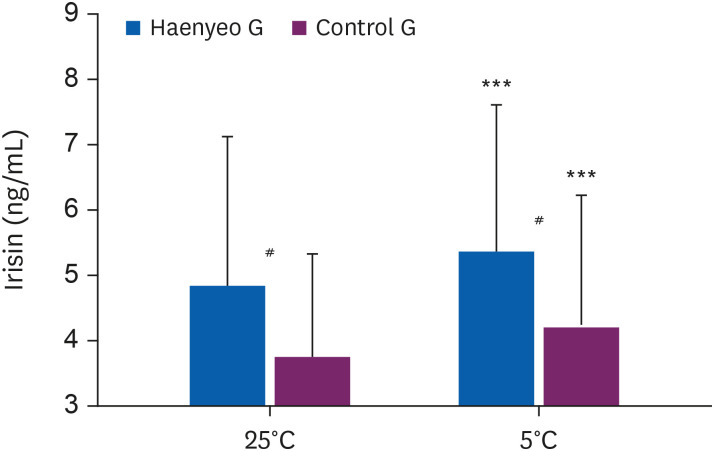
Irisin levels (Haenyeo G vs. Control G). Comparison of serum irisin levels before (pre) and after (post) cold stimulation (5°C ± 0.5°C). Values (Haenyeo G, n = 21 vs. Control G, n = 25) are presented as mean values ± standard deviation.
Haenyeo G: Haenyeo group; Control G: control group.
***p < 0.001, statistically significant differences between pre- and post-exposure values for Haenyeo G and Control G, respectively; calculated by paired t-test.
#p < 0.05, statistical differences in Haenyeo G and Control G values in pre-exposure, as well as post-exposure; calculated by independent t-test.
Before the experiment, irisin between the Haenyeo G and the Control G showed statistically differences (pre irisin levels in Haenyeo G vs. Control G, 4.83 ± 2.28 ng/mL vs. 3.73 ± 1.59 ng/mL, p < 0.05). Even after the experiment, there is a statistical difference in irisin between the Haenyeo G and the Control G (post irisin levels in Haenyeo G vs. Control G, 5.36 ± 2.23 ng/mL vs. 4.18 ± 2.04 ng/mL, p < 0.05).
DISCUSSION
Humans have the ability to detect and control body temperature for survival, but deviating from 37°C ± 3.5°C can lead to physiological dysfunction and life-threatening effects.26 Therefore, the human body has a heat generation reaction to maintain a constant body temperature, and there is a shivering mechanism and non-shivering heat generation mechanism.9272829 The function of these tissues decreases with age and recent studies showed that as aging progressed, thermogenesis decreased due to the decreased expression of UCP1 and ANS function decreased due to the loss of mitochondrial function in brown adipose tissue (BAT).303132
Orexin is involved in regulating body temperature in response to cold by promoting ANS related to heat generation (Fig. 1).121533 Orexin contributes to maintaining energy homeostasis as it drives the thermal mechanism in BAT.33 Orexin deficiency leads to thermal generation disorders in BAT, and obesity associated with orexin depletion was associated with energy degradation and low BAT activity.34 Therefore, the orexin system affects BAT function and acts like an orchestra that reacts together as the ANS is activated.153435 The increase in orexin induces browning in subcutaneous white fat in cooperation with irisin secreted by muscles such as during tremors and exercise, which is the result of the shivering mechanism.35
Irisin is associated with shivering mechanism and induces other genes related to oxidation.17 It is involved in heat generation by increasing the activity of BAT.1735 UCP1 expression is used as an indicator of the heat production activation of BAT but there is a limitation because it cannot be analyzed by non-invasive methods in humans.1116 Therefore, this study examined the ability of Haenyeo to produce BAT and cold adaptation through a biomarker, which induces the browning of white adipose tissue through the expression of UCP1 as a strong factor activating the exothermic program and release of factors such as irisin in white adipose tissue.111635 As a result, it was found that irisin increased even under cold exposure conditions and had characteristics dependent on body temperature change.12
In this study, Tty and mTb of Haenyeo G and the Control G showed a decrease after the experiment (Figs. 3 and 4). In terms of the amount of change, Haenyeo G was only a change of 0.05% in Tty and 0.1% in mTb. However, the Tty of the Control G showed a change of 1.00% and mTb of 0.66%. This means that the Control G is relatively weak in cold resistance compared to the Haenyeo G in terms of body temperature change. In addition, in comparing the Haenyeo G and the Control G, it can be seen that there was no statistical difference between Tty and mTb before the experiment, while there was a statistically significant difference after the experiment (p < 0.01). This suggests that Haenyeo have an advantage in surviving the cold.37
These characteristics are also evident in the results of orexin and irisin. First, the orexin and irisin levels are statistically higher in the Haenyeo G than in the Control G, which is the same not only before but also after the experiment (Figs. 5 and 6). In other words, in pyrogenes related to body temperature control, the Haenyeo G is basically higher than the Control G and is advantageous in coping with the risk of cold.12141535 Second, after the experiment, both orexin and irisin increased statistically significantly, which is the same for both Haenyeo G and Control G (Figs. 5 and 6). However, the important point is that there is a difference in the amount of change. Orexin increased by 6.41% in the Haenyeo G, but 18.13% in the Control G. Irisin increased by 10.97% in the Haenyeo G, and 12.06% in the Control G. This means that the change in pyrogens in the Control G was more pronounced compared to the Haenyeo G. Taken together, it can be seen that the Control G has a relatively large change in orexin and irisin levels compared to the Haenyeo G, and the Haenyeo can cope with cold stress only with a relatively small change in pyrogens in body temperature control.
In a previous study on pyrogens such as fibroblast growth factor-21 (FGF21) and irisin changes in one-time low-water temperature exposure in occupational scuba divers exposed to prolonged intermittent cold, plasma FGF21 increased significantly during cold exposure, whereas there was no difference in irisin levels compared before and after the experiment.36 This suggested the possibility of having a greater influence on body temperature control by non-shivering heat generation than heat generation by shivering during cold exposure.36 This is because FGF21 is involved in a non-shivering mechanism, but irisin is known to be associated with a shivering mechanism.3536 In this study, the increase amount of irisin was similar when comparing the Haenyeo G and the Control G, while the increase amount of orexin in the Control G was 2.83 times greater than the Haenyeo G. This would suggest that there is a greater possibility for the general public to have a greater influence on body temperature control by non-shivering heat generation than heat generation by shivering when controlling body temperature.
This difference was due to the special occupational history of Haenyeo. In this study, the Haenyeo G was women at an average of 68 years old, with a career history of more than 50 years, and who stayed at sea for 170–200 minutes an average of 28 days a month from March to August and more than 15 days a month in winter.4 These results mean that Haenyeo with more than 50 years of job experience have been continuously exposed to a cold environment in the sea and adapted to it as professionals.7 This study confirmed that both orexin and irisin in the blood affected the formation of BAT in Haenyeo who were continuously exposed to low temperatures over a long time.6101114
Taken together, Haenyeo have a better defense system against cold stress, which is seen as equal core temperature, lower Tsk, reduced heat loss from skin, and less energy consumption to adapt to cold environments.367 Underwater and hyperbaric environments include hazards to divers, particularly hypothermia, and underwater diseases can be known by a diving medicine physician (DMP).37 For accurate evaluation of divers working in underwater spaces, not only scuba, but also breathless divers such as Haenyeo, DMP must have occupational understanding and knowledge of environmental hazards.3738 OEM is a medical field that deals with physical and biological hazards and assessment of fitness to work.37 In this regard, it is necessary to define the health standards of divers and determine the physiological response so that the work can be carried out safely.3738 For this reason, it seems that DMP should be OEM specialized. In particular, in order to expand the research area of OEM in body temperature control in an underwater environment, research on factors related to heat generation and energy metabolism in the future in addition to orexin and irisin should be conducted.394041
Therefore, the low-temperature purification data of Haenyeo is meaningful from the perspective of OEM for maintaining body temperature and cold diseases of workers working for a long time in low-temperature environments. However, there were several limitations to this study.
First, this study targeted female divers with more than 50 years of professional experience, and the number of study participants was not large. In terms of elderly subjects, it was not easy to secure the number of subjects due to the possibility of physical problems.
Second, in this study, the Haenyeo G and the Control G were compared in consideration of only some of the biomarkers related to thermal generation. In addition to orexin and irisin, biomarkers of BAT such as FGF21 should be fully investigated in future studies.353640 In fact, it is most accurate to check the presence or absence of BAT with positron emission tomography (PET)/computed tomography (CT), but it is not easy to implement due to economic and methodical problems. In the future, research on the relationship between PET/CT results and pyrogens should also be conducted.42
Nevertheless, this study is meaningful in that it was the first to obtain data on body temperature changes for Haenyeo’s cold adaptation and basic medical data on orexin and irisin, which are known to be involved in body temperature control compared to the ordinary person.
CONCLUSIONS
This study explored the occupational physiological characteristics of Haenyeo in low-temperature stress conditions developed by chronic low-temperature exposure. The results suggest that the Haenyeo G were relatively superior to the Control G in inducing heat generation. This means that the tolerance to cold is higher than that of the Control G, and Haenyeo are advantageous for cold survival.
Notes
Funding: The authors thank the study subjects whose participation made this study possible. This research was supported by a grant (No.2016R1D1A3B02015394) from the Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Education, Republic of Korea.
Competing interests: The authors declare that they have no competing interests.
Author contributions:
Conceptualization: Lee I, Lee JB.
Data curation: Lee I, Lee JB, Lee YJ, Jang EC, Kwon SC, Min YS, Yun J, Park T, Choo E.
Formal analysis: Lee I, Lee JB, Lee YJ, Jang EC, Kwon SC, Min YS, Yun J, Lee HJ, Choo E.
Investigation: Lee I, Lee JB, Lee YJ, Jang EC, Kwon SC, Min YS, Park T, Lee HJ, Choo E.
Writing - original draft: Lee I.
Writing - review & editing: Lee JB.
Abbreviations
ANS
autonomic nervous system
BAT
brown adipose tissue
BMI
body mass index
BMR
basal metabolic rate
BP
blood pressure
Control G
control group
CT
computed tomography
DMP
diving medicine physician
ELISA
enzyme-linked immunosorbent assay
FGF21
fibroblast growth factor-21
Haenyeo G
Haenyeo group
HR
heart rate
mTb
mean body temperature
OEM
occupational environmental medicine
PET
positron emission tomography
Tsk
skin temperature
Tty
tympanic temperature
UCP1
uncoupling protein 1
VO2
oxygen consumption