Asbestos exposure and autoantibody titers
Article information
Abstract
Background
Asbestos is a well-known hazardous substance that causes occupational and environmental diseases including asbestosis (lung fibrosis). Silica exposure which causes silicosis (another type of lung fibrosis) has long been linked to the development of autoimmune diseases; however, there are few studies on the relationship between asbestos exposure and autoimmune diseases.
Methods
A total of 54 individuals who had worked in a former asbestos textile factory underwent autoantibody-related blood tests, chest X-ray imaging, and pulmonary function tests. Based on the job exposure matrix (JEM), the estimated asbestos exposure concentrations were determined, and the presence of asbestosis was determined by chest radiography.
Results
Scleroderma (Scl-70) and ribonucleoprotein (RNP) antibodies were significantly lowered in the pleural plaque present group than in the absent group. Additionally, Scl-70, RNP, and Sjögren's syndrome type B (SS-B) antibodies were significantly lowered in the asbestosis present group. When stratifying variables with or without asbestosis, Scl-70, Smith, SS-B, and RNP antibodies decreased in female, crocidolite handling group, and higher estimated asbestos exposure level group.
Conclusions
Contrary to our expectations that autoantibody titers would be higher in groups with high asbestos exposure or in the asbestosis group, those with asbestosis showed lower titers. But as our research has some methodological limitations, the lowered titer of autoimmune antibody in our asbestos exposed subjects could not be simply interpreted as a lowered risk of autoimmune diseases. So careful interpreting should be taken when examine autoantibodies to screening or diagnose autoimmune diseases in people with asbestos exposure. In addition, it is necessary to establish relevance of asbestosis and autoantibodies through further studies of larger scale and higher confidence levels.
BACKGROUND
Asbestos is a fibrous mineral obtained from nature, and has been used around the world for its unique properties and low price [1]. However, as carcinogenicity and harmfulness of asbestos have been revealed, its use has been banned worldwide. In 1987, the International Cancer Institute (IARC) designated asbestos as a group 1 with sufficient evidence of carcinogenicity in humans [2]. Asbestos-related diseases (ARDs) caused by occupational and environmental asbestos exposure include malignant mesothelioma, lung cancer, asbestosis, and pleural disease. In addition to the effects on the lungs, the association with asbestos has been found in diseases such as the peritoneum, immune system, digestive system, and reproductive system [3].
There have been a lot of research on the harmful effects of silica; it is physically and chemically similar to asbestos and can cause lung fibrosis (silicosis). Exposure to silica is known to increase antinuclear antibodies (ANA) and the risk of systemic lupus erythematosus (SLE) [4], rheumatoid arthritis (RA) [5], and systemic sclerosis (SSc) [6] in both mice and humans [7]. While the relationship between silica exposure and some specific systemic autoimmune diseases (SAIDs) has been fairly well accepted, correlations between asbestos exposure and immune-related diseases are still insufficient [8].
The autoantibodies refer to antibodies that are produced by immune response against the body's own self antigen. These may be present years before the diagnosis of a certain autoimmune diseases, so it can be used for early prediction combining with genetic information and family history [9,10]. However, the presence of autoimmune antibodies does not always mean that there are autoimmune diseases, and low level of autoimmune antibodies may also be present in healthy individuals [11]. In general, rheumatoid factor (RF) and ANA tend to increase in various rheumatoid diseases including rheumatoid arthritis, SLE etc. Anti-centromere antibodies (ACAs) are mainly associated with inflammatory diseases of small blood vessels. Anti-Sm antibodies and anti-ribonucleoprotein (RNP) are more specific for SLE. Thus, Sjögren's syndrome type A (SS-A/Ro) and Sjögren's syndrome type B (SS-B/La) usually increase in Sjögren's syndrome, and Scl-70 increases in diffuse SSc or Calcinosis, Raynaud Phenomenon, Esophageal Dysmotility, Sclerodactyly, and Telangiectasia (CREST) syndrome [12,13].
The results of research on the effect of asbestos exposure on autoantibodies were inconsistent. Previous research on asbestos exposure had been done in Libby, MT, USA. Residents here were known to be exposed to asbestos both occupationally and environmentally [14]. Results of serological tests showed higher ANA positive rates for residents in Libby compared to control group. ANA titer and IgA levels were also significantly higher [15]. In Western Australia's Wittenoom mining site where the asbestos mine is located, and the mortality rate from malignant mesothelioma is high [16], an analysis of the ANA in this cohort showed that Wittenoom residents were approximately 5 times more ANA positive than control group, and the ANA titer was also high [17]. Additionally, in the population-based case-control study of the Swedish population, asbestos exposure increased the risk of seropositive RA (odds ratio [OR]: 1.2, 95% confidence interval [CI]: 1.0–1.4) and seronegative RA (OR: 1.2, 95% CI: 1.0–1.5) in male workers [18]. Also, the high frequency of positive RF in asbestos workers compared to the general population has been reported in several past studies [19-21]; however, other studies have reported no association [15,22,23].
On the basis of these previous findings, we conducted a study to identify the relationship between asbestos exposure and autoimmune diseases by examining autoantibodies in asbestos-exposed groups in Korea. We investigated the correlations between each asbestos exposure scenario and autoantibody titers of workers in asbestos textile factory, which shows the highest occupational asbestos exposure level in Korea.
METHOD
Study subjects
The study subjects were people who worked at J Chemical, the largest asbestos textile factory which was located in Busan, Republic of Korea. They were issued an ‘Health Management Pocketbook’ on asbestos by Korea Occupational Safety & Health Agency (KOSHA) and underwent ‘Regular Medical Examination of Pocketbook Holder’ annually at Pusan National University Yangsan Hospital environmental health centers for asbestos (EHCA). None of the subjects were previously diagnosed any autoimmune disease at the time of examination. Finally, a total of 54 subjects were included in the study. Appropriate informed consent was obtained from all subjects.
Serological and other functional examination
From September 27, 2010 to October 2, 2010, the study participants were tested serologically, chest X-ray imaging, and pulmonary function tests (PFTs). The serum sample was obtained total 3.4 mL using plain tube or SST tube, then analyzed by chemistry or enzyme-linked immunosorbent assay kit analyzer. The titer was evaluated quantitatively because the percentage of participants who were positive for autoantibodies was very low (RF: 11%, ANA: 3.7%, ACA and RNP: 1.9%, 0% other autoantibodies were all negative). The results below the detection limit were treated as zero.
Posteroanterior chest radiography was performed using an FCR 5501 digital radiograph device (Fuji, Tokyo, Japan). The radiographs were interpreted by 2 separate radiologists according to the ARD guidelines of the Korean Society of Thoracic Radiology. The results were divided into 2 groups based on the presence or absence of asbestosis. Also, the results for pleural plaque also divided into 2 groups: the presence or absence of the pleural plaque.
The PFT was performed using Vmax series 229 spirometry (SensorMedics, Yorba Linda, CA, USA) by an experienced examiner who has passed the special medical examination pulmonary function quality. For the acceptability and repeatability, the tests were repeated at least three times and the best results were chosen. The results were divided into 3 groups: normal, restrictive ventilatory defect, and obstructive ventilatory defect. The participants were classified into restrictive ventilator defect group when forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ≥ 70% and FVC < 80% of mean predicted value, and classified into the obstructive ventilator defect group when FEV1/FVC < 70% of mean predicted value. When FEV1/FVC ≥ 70% and FVC ≥ 80% of mean predicted value, the participants were classified into normal group. In the case of mixed obstructive and restrictive ventilator defect pattern, there were no subjects and they were excluded.
Asbestos exposure profile
The detailed occupation-related asbestos exposure history was confirmed through a structured questionnaire [24]. The questionnaire was completed through one-on-one interviews with trained researchers. It consists work periods, latent periods, specific task they did, and the type of handling asbestos. The specific tasks they did were carding, twisting, weaving, spinning, gasket, fixing, managing, packaging, etc., and the type of handling asbestos were chrysotile, crocidolite, and both. The work period was divided into 4 groups: 0–1 years, 2–4 years, 5–9 years, and more than 10 years. We defined the latent periods as the time from retirement to the test date, we also divided it into 4 groups: 0–10 years, 11–20 years, 21–30 years, and 31–40 years. By integrating such information, estimated exposure level of asbestos were calculated using linear equations based on job exposure matrix (JEM) [25]. We divided it into 3 groups according to asbestos exposure level: less than 1 f/cc, 1–10 f/cc, more than 10 f/cc.
Data analysis
The statistical analysis was performed using SPSS Statistics for Windows, version 25 (SPSS Inc., Chicago, USA). Data were expressed as geometric means and geometric standard deviations. The statistical significance was tested based on p < 0.05 significance level, using Mann-Whitney U test or Kruskal-Wallis test.
RESULTS
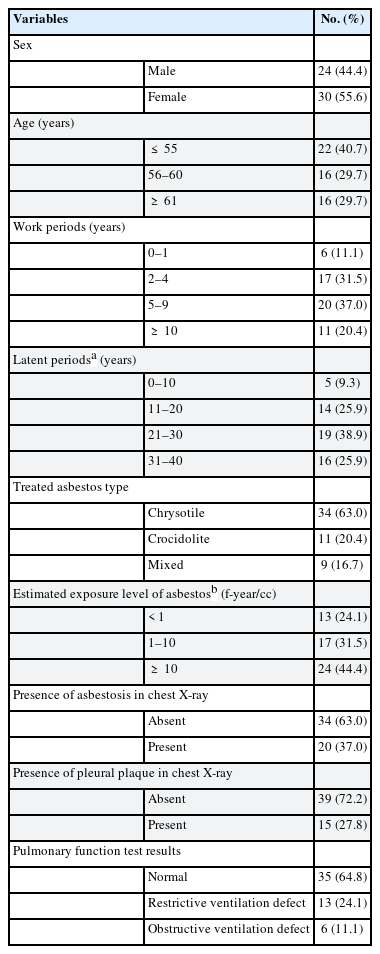
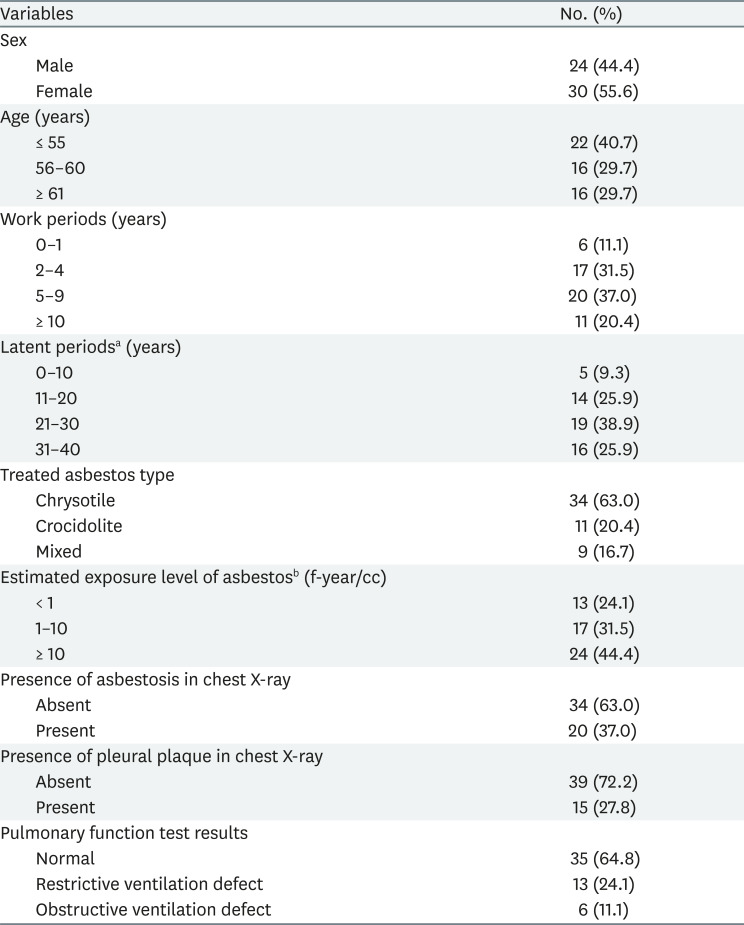
Table 1 shows the general characteristics of the subjects, the factors related to past asbestos exposure, and the distribution of clinical findings. The subjects were 55.6% women and 44.4% men. The average age of the subjects was 57.3 years. The average working years was 5.8 years, and the average latent period was 24.2 years. The chrysotile-handling group was the largest (63.0%), followed by the crocidolite-handling group (20.4%), then the both chrysotile and crocidolite-handling group (16.7%). Estimated asbestos exposure level was 2.3 f-year/cc on average. Chest X-ray revealed asbestosis in 37% and pleural plaque in 27.8% of the subjects. In PFT, 64.8% were normal, 24.1% had restrictive ventilation defect, and 11.1% had obstructive ventilation defect.
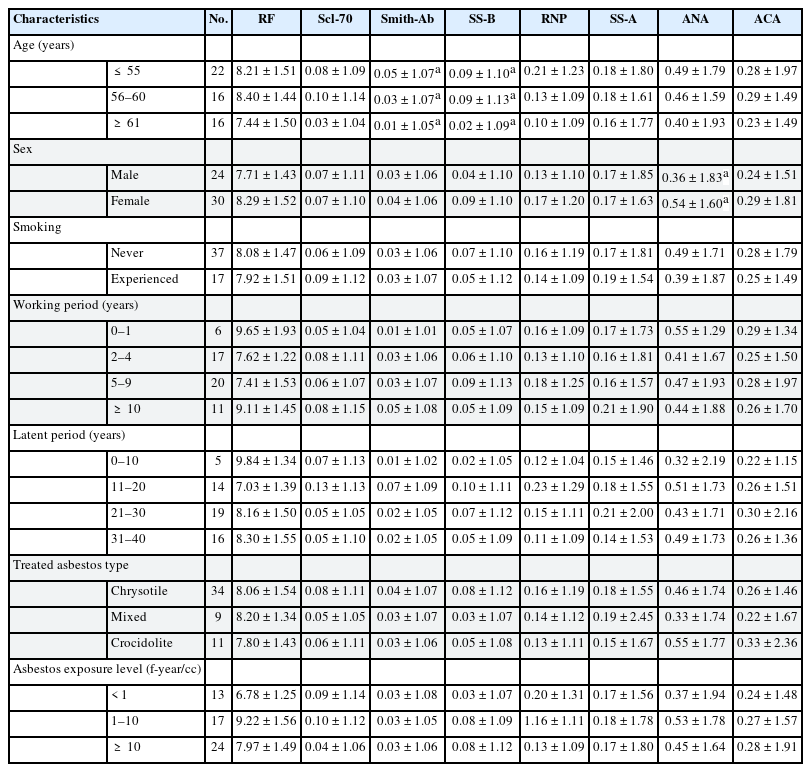
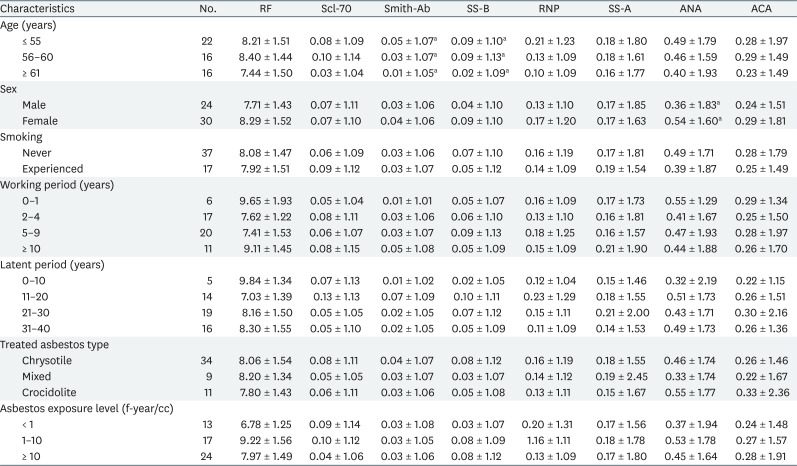
Table 2 shows the distribution and relation of each autoantibody with general characteristic variables and the occupational exposure related variables. The variable that showed a significant difference among subgroups for Smith was age, it was significantly decreased as age increased. Likewise, SS-B also decreased significantly with increased age. ANA titer was significantly higher in females than in males. Smoking history, work period, latent period, types of handling asbestos, and levels of asbestos exposure did not show significant association with autoantibody titers.
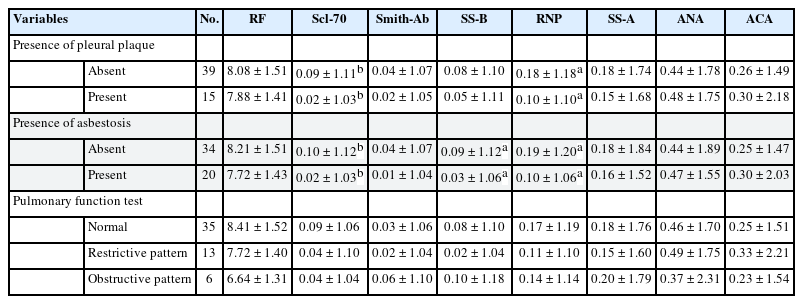
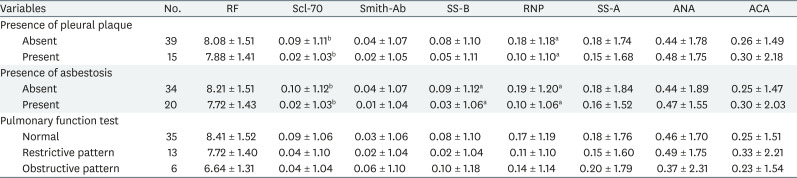
Similarly, Table 3 shows the distribution and relationship between each autoantibody and the clinical findings. Scl-70 and RNP were significantly lower in the pleural plaque or asbestosis group than in the normal group. In the group with asbestosis, SS-B titer was also low. No autoantibody was found to be significantly associated with PFT results.
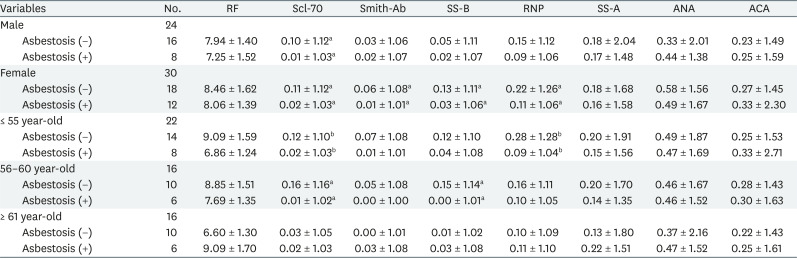
We conducted stratification analysis on sex, age, type of handling asbestos, and asbestos exposure levels to identify the changes in autoantibody titers with or without asbestosis. Consequently, Scl-70, Smith, SS-B and RNP showed inverse correlation in females with asbestosis, and Scl-70 also showed significant correlation in males with asbestosis. At age 55 and below, Scl-70 and RNP were significantly lowered in the asbestosis group. Between age 56–60, Scl-70 and SS-B were inversely correlated. However, there was no autoantibody associated with asbestosis in patients older than 61 years (Table 4).
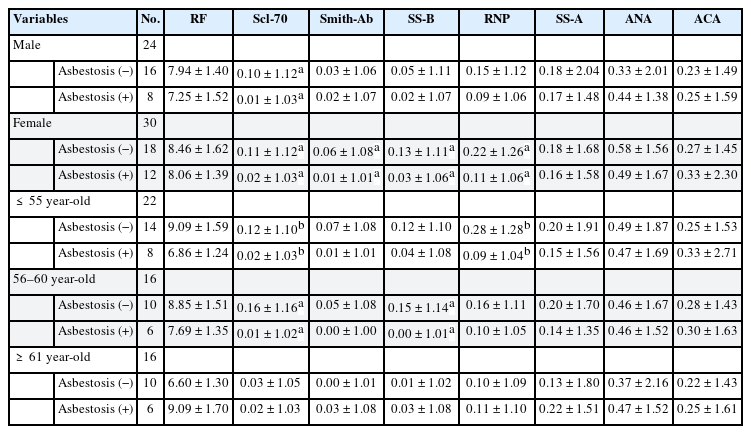
Presenence of autoantibodies by sex and age based on the presence or absence of asbestosis (Geometric mean ± geometric standard deviation)
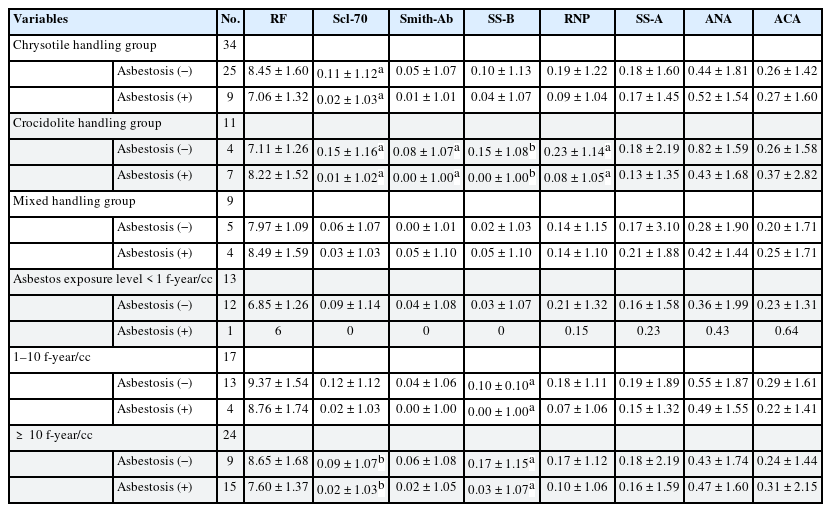
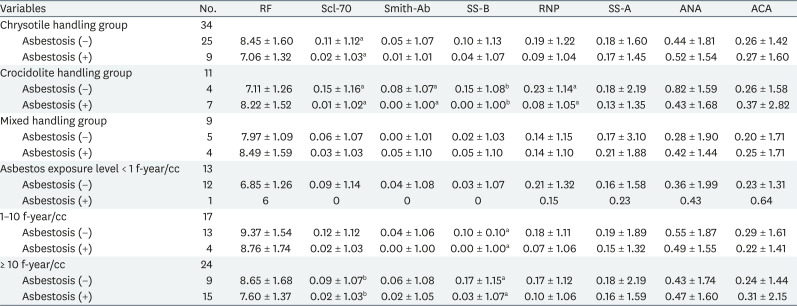
In Table 5, the Scl-70 titer was significantly lowered for the chrysotile-handling group with asbestosis; the titer was also lowered in Scl-70, Smith, SS-B, and RNP for the crocidolite-handling group with asbestosis. In both chrysotile and crocidolite handling groups, there was no relevant autoantibody with or without asbestosis. Asbestos exposure levels were significantly decreased in Scl-70 in the asbestosis group over 10 f-year/cc and SS-B tended to decrease significantly with asbestosis in the 1–10 f-year/cc and over 10 f-year/cc exposed groups. However, in the 1 f-year/cc exposed group, there was only one patient with asbestosis; hence, these results should be cautiously interpreted.
DISCUSSION
The present study investigated the relationship between asbestos exposure and autoimmune diseases. We identified autoimmune antibody titers to predict the development of autoimmune diseases. We classified the participants to several subgroups according to general characteristics, asbestos exposure-related characteristics, chest X-ray, and PFT results to identify the differences in the level of autoimmune antibodies in each variable. Interestingly, contrary to our expectations that autoantibody titers would be higher in high asbestos exposure group or the asbestosis group, some of those with asbestosis showed significantly lowered titers. Scl-70, Smith, SS-B, and RNP showed significantly lowered titers in women with asbestosis and crocidolite handling group with asbestosis. Additionally, at age 55 and below, Scl-70 and RNP were significantly lowered in the asbestosis group, and in case of age 56–60 group, Scl-70 and SS-B were lowered in the asbestosis group. Furthermore, Scl-70 was significantly decreased in those with over 10 f-year/cc asbestos exposed group with asbestosis and SS-B were decreased significantly in the 1–10 f-year/cc and over 10 f-year/cc exposed groups with asbestosis.
Silica has been studied more than asbestos, and there are well-known complications in patients with silicosis including RA, SSc, SLE, and ACA-related vasculitis. It is also known that ANA, Scl-70, and ACA are often found in this condition, even when symptoms related to autoimmune diseases have not appeared [26]. Despite the extensive epidemiologic studies on the association between silica and systemic autoimmune diseases, the exact mechanism is yet unknown. In case of asbestos, it is more difficult to draw conclusions because of the limited number of epidemiological studies between asbestos exposure and autoimmune disease. But the previous studies have revealed that some autoantibodies tend to more present in the asbestos-exposed group even without autoimmune diseases [8,14-20]. Few studies have investigated the relationship with asbestos and the titers of antibodies, but these have shown increased RF or ANA titers in asbestos-exposed groups [14,15,17,19-21,27]. These studies have also shown that higher ANA positive rates in groups with asbestos exposure were associated with severity or faster progression of lung disease [15,28-31]. Although these studies suggest that autoantibody production can be caused by tissue damage from lung disease, Tamura et al. [29,31] found that autoantibodies existed prior to lung disease in most cases. Another study suggests that autoantibodies are not a nonspecific consequence of pulmonary fibrosis because of the absence of autoantibodies in other chronic fibrotic lung diseases [28].
The most likely mechanism for increasing autoantibodies in asbestos-exposed people is first taken up by macrophages when asbestos fibers enter the body. These macrophages release ROS and cause inflammation through inflammasome activation, and inflammation is accelerated by promoting inflammatory cytokine secretion. Autoimmune antigens produced by apoptosis of macrophages are presented to T cells through APC, and finally, autoimmune antibodies are produced from B cells [8,32]. Recent studies have suggested that inflammasome activation by asbestos may promote proinflammatory effects such as interleukin-1β release [33,34]. It was also founded that chrysotile and amphibole both activate caspase cascade, oxidative stress, and Nod-like receptor family protein 3 (NLRP3) inflammasome; however, there were some differences in specific pathways between the 2 fibers [35].
In the present study, all subjects had no history of autoimmune disease diagnosed at the time of examination, and few patients showed positive autoantibodies. Two of the positive participants were diagnosed with autoimmune-related diseases years later, one with p-ACA associated rapid progressive glomerulonephritis (RPGN) and the other with ANA-positive undifferentiated connective tissue disease (UCTD). Their estimated asbestos exposure concentration was at the highest level (more than 10 f/cc). One had diagnosed with asbestosis at the time of examination, and the other was normal, but later diagnosed with asbestosis. They were all diagnosed with autoimmune disease after being diagnosed with asbestosis. This finding is consistent with previous findings that high concentrations of asbestos exposure can be related to positive autoantibodies or autoimmune diseases. However, our study results showed that some autoantibody titer was lowered in participants with asbestosis, which means a strong evidence of asbestos exposure.
Autoantibodies that were shown to decrease in relation to asbestosis were mainly Scl-70, SS-B, Smith, and RNP. Anti-Scl-70 antibody, also called anti-topoisomerase I [36], is an antibody found in diffuse SSc or CREST syndrome; it is highly related to severe scleroderma disease [37]. In the presence of Scl-70 autoantibody, the incidence of pulmonary fibrosis is high and mortality is known to be higher. Anti-SS-A/Ro antibodies and anti-SS-B/La antibodies are representative autoantibodies related to Sjögren's syndrome, a chronic rheumatic disease characterized by progressive dryness of the eye, mouth, and other mucosal tissues; it is also accompanied by other rheumatoid diseases such as RA and SLE [38]. Anti-Sm antibody and anti-RNP antibody are autoantibodies that are specific for autoimmune diseases, especially SLE. Anti-Sm antibodies are known to be detected in 15%–30% of SLE patients, and anti-RNP antibodies are found in 30%–40% of SLE patients. These kind of autoantibodies have never been studied for their relevance to asbestos. Also, the relationship between these autoantibodies related autoimmune diseases has been rarely studied. Only a study in lupus or scleroderma patients has found a relationship to asbestos exposure [39], and other study has shown an increased risk of SSc related death in people with occupational asbestos exposure [40]. Furthermore, there have not been identified the clinical significance of low antibody titers of autoantibodies.
Our study did not prove the hypothesis that higher autoantibody frequencies and autoimmune diseases would positively associated in asbestos-exposed groups. On the contrary, the negative correlation was observed between several autoantibodies and asbestos exposure scenario. This is thought to be an accidental discovery due to research limitations. In practice though, there can be a certain mechanism in which the autoantibody titer decreases in the early stages of the disease, but there have been no previous studies to prove it, and it is reasonable to interpret it as a negative study according to biological plausibility. So, the lowered titer of autoimmune antibody in our asbestos exposed subjects could not be simply interpreted as a lowered risk of autoimmune diseases.
One limitation of this study is that the small and limited sample size may have resulted in the dropout of autoimmune disease or severe asbestosis patients, who may not have been included in the study. Therefore, the inclusion of these groups may lead to different results. In addition, the autoimmune diseases that were significantly associated with autoantibodies in our study have a very low prevalence and incidence. Although the prevalence of RA in the general population is relatively high at 860 per 100,000 persons, the prevalence is very low for SLE (32 per 100,000 persons), SSc (24 per 100,000 persons), and Sjögren's syndrome (14 per 100,000 persons). Considering the long-term morbidity of autoimmune diseases, the incidence for autoimmune diseases is likely to be lower. In the group exposed to asbestos, the prevalence of SAIDs seems to be so small that few studies have been conducted on SAIDs and asbestos exposure. Moreover, although relatively many RA studies have been conducted, the results of these studies were inconsistent. Therefore, it is difficult to determine the actual prevalence and incidence for autoimmune diseases in the asbestos exposed group. Nonetheless, patients exposed to asbestos are thought to also have a low prevalence and incidence for autoimmune diseases. The small sample size and the epidemiological characteristics of autoimmune diseases may contribute to limitations in deriving research results. Furthermore, women account for a much higher proportion of the population with autoimmune diseases (SLE: 88%, SSc: 92%, RA: 75%, Sjögren's syndrome: 94%) [41]. The previous studies which investigated asbestos and autoantibody were mainly men with occupational asbestos exposure. But in our study, the proportion of women is high and there may be differences according to the study subjects' general characteristics.
Secondly, in our study, asbestosis was diagnosed based only on routine chest X-ray examinations, which have low validity and reliability. The diagnostic criteria for asbestosis in Korea are based on computed tomography findings and criteria provided by a Korea environment corporation. Hence, these diagnostic criteria may differ greatly from the diagnostic criteria based on simple chest radiography, which may have contributed to diagnostic errors in this study. In addition, the possibility of erroneously diagnosing interstitial lung disease associated with autoimmune disease as asbestosis could not be excluded. In general, interstitial lung disease tends to increase in autoimmune diseases. However, in such a case, a positive correlation between autoantibodies and interstitial lung disease should appear, but this study showed the opposite. It is necessary to review the results of this study through additional examination modality such as chest computed tomography and additional analysis accordingly.
Thirdly, a different type of asbestos may have been exposed compared to previous studies. In previous studies, Libby's is mainly composed of tremolite, while Witternoon's is composed of crocidolite, and their subjects were exposed to asbestos mixed with other minerals from the asbestos mine. Our study subjects handled purified pure chrysotile and crocidolite for industrial usage; hence, the difference may have occurred here. Finally, since high proportion of the test results were at the subclinical level, it is difficult to evaluate the clinical meaning of the test itself. Furthermore, the analysis of the association with autoantibodies could not confirm the direct association with autoimmune diseases. Therefore, it is necessary to establish relevance of asbestosis and autoantibodies through further studies of larger scale and higher confidence levels. Additionally, in countries where asbestos is still in use, strict sanction is needed to completely ban the use of asbestos. Considering the harmfulness and long latency of asbestos, further continuous research on high asbestos exposure groups are necessary.
CONCLUSION
The results did not show that asbestos exposure and autoantibodies were positively related. Although some autoantibodies were inversely associated with higher asbestos exposure groups, the clinical significance is yet uncertain because lack of previous studies about asbestos and autoantibodies which shows valid results in our study, and limitations of our research method. Also, this study is a limited-scale cross-sectional study, so that it is difficult to generalize the results. This suggests that careful interpreting should be taken when examine autoantibodies to screening or diagnose autoimmune diseases in people with asbestos exposure. Therefore, it is necessary to establish relevance of asbestosis and autoantibodies through further studies of larger scale and higher confidence levels. Furthermore, international sanction of asbestos-using countries and continuous attention to the high asbestos exposed group are needed.
Notes
Competing interests: The authors declare that they have no competing interest.
Author Contributions:
Conceptualization: Kang D.
Data curation: Lee E.
Formal analysis: Lee E, Kang D.
Investigation: Lee E, Kang D.
Writing - original draft: Lee E.
Writing - review & editing: Lee E, Kang D, Kim Y, Kim SY.
Abbreviations
ACA
anti-centromere antibody
ANA
antinuclear autoantibodies
ARDs
asbestos-related diseases
IARC
The International Cancer Research Institute
JEM
job exposure matrix
NLRP3
Nod-like receptor protein 3
PFT
pulmonary function test
RA
rheumatoid arthritis
RF
rheumatoid factor
RNP
Ribonucleoprotein
RPGN
rapid progressive glomerulonephritis
SAIDs
systemic autoimmune diseases
Scl-70
Scleroderma-70
SLE
systemic lupus erythematosus
SS-A
Sjögren's syndrome type A
SS-B
Sjögren's syndrome type B
SSc
systemic sclerosis
UCTD
undifferentiated connective tissue disease