Validation of urinary 1,2-dichloropropane concentration as a biological exposure index for workers exposed to 1,2-dichloropropane
Article information
Abstract
Background
The International Agency for Research on Cancer classified 1,2-dichloropropane (1,2-DCP) as a human carcinogen in 2016. It is necessary to establish a health monitoring system for workers exposed to 1,2-DCP. We investigated the correlation between 1,2-DCP concentration in air and urine to determine whether it is appropriate to measure 1,2-DCP in urine as a biological exposure index (BEI).
Methods
Twenty-seven workers from 3 manufacturing industries handling 1,2-DCP participated in this study. Airborne 1,2-DCP was collected by personal air. Urine samples were collected at the end of work and analyzed using gas chromatography-mass spectrometry. Correlation analysis and simple regression analysis were performed to investigate the relationship between 1,2-DCP concentration in urine and air.
Results
Pearson correlation coefficients between total 1,2-DCP in air and urine (uncorrected, creatinine-corrected) were 0.720 and 0.819, respectively. For urine samples analyzed within 2 weeks, the Spearman's rho of 1,2-DCP concentration in urine (uncorrected and creatinine-corrected) was 0.906 and 0.836, respectively. Simple regression analysis of 1,2-DCP in air and urinary 1,2-DCP concentrations within 2 weeks, which showed the highest correlation, revealed that the coefficient of determination of 1,2-DCP concentration in urine (uncorrected and creatinine-corrected) was 0.801 and 0.784, respectively.
Conclusions
As a BEI for workers exposed to 1,2-DCP, urinary 1,2-DCP without creatinine correction better reflects the exposure levels of 1,2-DCP in air.
BACKGROUND
Cleaning operations are widely employed in machinery, metal, electronic, automotive, precision instrument, glass and optical, surface treatment and plating, and other (plastics, rubber, chemicals, printing, and cosmetics) industries. With the recent lowering of exposure limits for trichloroethylene (TCE) used in cleaning operations (in 2016, the public notice of the Korean Ministry of Employment and Labor changed the time-weighted average for TCE from 50 ppm to 10 ppm), there is a trend of replacing TCE with unregulated substances in workplaces [1]. According to the report of the 2014 Work Environment Investigation conducted by the Korea Occupational Safety and Health Agency, the number of 1,2-dichloropropane (1,2-DCP) handling workplaces has gradually increased from 35 in 2009 to 343 in 2014 [2]. According to the chemical investigation result of the Ministry of Environment, 186 sites use and handle 1,2-DCP, of which 38 sites use and handle more than 20 tons of 1,2-DCP a year [3].
Acute exposure to 1,2-DCP affects respiratory, digestive, hematopoietic, renal, dermal, ocular, nervous, and hepatobiliary systems [4]. Regarding the health effects of acute exposure to 1,2-DCP, Rubin [5] described an event in which 2,000 gallons of 1,2-DCP leaked during an accident, affecting hundreds of people who were exposed by inhalation. People who were exposed to 1,2-DCP complained of upper respiratory system irritations such as chest discomfort, difficulty breathing, and coughing. Pozzi et al. [6] reported that hemolytic anemia and disseminated intravascular coagulation occurred in one case of oral ingestion of 1,2-DCP and 2 cases of inhalation exposure owing to an accident. Elevated creatinine levels, elevated blood urea nitrogen levels, oliguria, and hematuria were observed in people exposed to 1,2-DCP by inhalation and acute tubular necrosis was noted in a kidney biopsy. Lucantoni et al. [7] reported elevated liver enzyme levels in workers exposed to stain removers containing 1,2-DCP and centrilobular necrosis in liver biopsies. There is also a case report of acute toxic encephalopathy caused by acute exposure to 1,2-DCP [8]. Chronic exposure to 1,2-DCP increases the risk of central nervous system disease and cholangiocarcinoma. According to a standardized incidence ratio study of cholangiocarcinoma conducted in workers exposed to 1,2-DCP by Kumagai et al. [9], the risk of cholangiocarcinoma significantly increases with increased concentration and cumulative exposure. The International Agency for Research on Cancer (IARC) classified 1,2-DCP as a human group 1 carcinogen in 2016 [10]. It is necessary to establish a health monitoring system for workers exposed to 1,2-DCP.
In this study, we investigated the relationship between 1,2-DCP concentration in air and urine to determine the validity of 1,2-DCP concentration in urine as a biological exposure index (BEI) for workers exposed to 1,2-DCP. This may provide a basis for the establishment of an accurate monitoring system.
METHODS
Study population
Work environment measurement of 1,2-DCP in workers from 7 workplaces was conducted for the “Cleaning agents including 1,2-DCP handling status investigation and health monitoring system establishment” research by the Occupational Safety & Health Research Institute. When we used G*Power for calculation of appropriate sample size with effect size (correlation coefficient) 0.5, α-error 0.05, and power 0.8, the appropriate sample size was 29 [11]. So we recruited 29 1,2-DCP exposed and 4 non-exposed office workers, and took informed consent from participation. The urinary 1,2-DCP levels in 4 office workers were under detection limit, so they were excluded at the analyses. The participants were men with an average age of 35 years and with no underlying diseases. All workers wore gloves as personal protective equipment during work but none used masks during operation. The workers were engaged in the printing industry, automobile parts manufacturing industry, plating industry. The working hours are 8hours during the day.
1,2-DCP concentration in air and urine
The collection medium comprised a charcoal tube (100 mg/50 mg; SKC Inc., Covington, GA, USA) connected to a low-flow pump (Gilian LFS-113; Sensidyne, St. Petersburg, FL, USA) and a flow calibrator (Defender 510-M; Bios International Corp., Butler, NJ, USA) used at a flow rate of 0.05 L/min. Calibration of flow rate was performed before and after sampling. The samples were sealed with a stopper and stored in a dark environment at 25°C until analysis according to the Sampling Stability of Analysis Method #1013 from the National Institute for Occupational Safety and Health [12]. The samples were analyzed using a gas chromatography-flame ionization detector (CP-3800; Varian Inc., Palo Alto, CA, USA). The detection limit was 0.03 ppm.
To evaluate the average exposure level of workers, the method of personal sampling was used. The measuring device was positioned over the mouth and nose. The measurements were continuously taken for 6 hours or more during the working hours in a single day or by dividing the work hours into equal intervals for continuous measurement of 6 hours or more. After 6 hours of measurement, the results were evaluated with the time-weighted average value. Urine samples were collected by workers in a 50 mL tube before 2 hours or at the end of work. An icebox was used to transfer the samples and they were stored frozen until analysis. As various substances are detected in urine owing to its characteristics, selective analysis for 1,2-DCP was required. Therefore, we approached the Korea Basic Science Institute (KBSI) for the selective analysis of 1,2-DCP using gas chromatography-mass spectrometry. KBSI is a government-funded research institute within the Ministry of Education, Science and Technology. It is a reputable research institute with ISO 9001 (Quality Management System) certification for analytical services.
Two milliliters of urine were added to a vial and solid-phase microextraction fiber was mounted onto the headspace autosampler. The analytical instrument used was the gas chromatography-MS detector (7890B/Triple Quad MS 7010/CTC PAL RSI 85; Agilent, Santa Clara, CA, USA). The standard concentration range was divided into 8 sections ranging from 0.5–250 µg/L. Samples exceeding the standard concentration range were diluted and analyzed again. The detection limit (S/N = 3) was 0.30 µg/L and practical quantification limit (S/N = 10) was 1.00 µg/L.
Statistical analysis
As the distribution of 1,2-DCP concentration in urine is right-skewed, log-transformation was performed so that data were normally distributed. The concentration of 1,2-DCP in urine (uncorrected, unit: µg/L and creatinine-corrected, unit: µg/g creatinine) was used to compare the degree of correlation. In addition, samples were divided according to duration from urine sampling to analysis: those analyzed within 2 weeks and those analyzed after 4 weeks.
A correlation analysis was performed to compare the correlation between 1,2-DCP concentration in air and urine samples obtained by personal sampling at the end of work. Pearson's correlation analysis was performed on total samples. Spearman's rank correlation analysis, a nonparametric test, was conducted on samples within 2 weeks and after 4 weeks since the number of those samples was not sufficient for parametric test. Furthermore, to determine whether period after frozen storage affects the results, a comparison for the 2 groups was performed by partial correlation analysis.
Simple regression analysis was performed to compare the 1,2-DCP concentration in urine with that in air, and the coefficient of determination (R2) of 1,2-DCP concentration in urine without correction and with creatinine correction was calculated. A p-value less than 0.05 was considered statistically significant. SPSS software 25.0 (IBM, Chicago, IL, USA) was used in all statistical analyses.
Ethics statement
This study protocol was approved by Institutional Review Board of Korea Occupational Safety and Health Agency (2017-IRB-06).
RESULTS
Distribution of 1,2-DCP by type of industries and processes
Seventeen workplaces in the manufacturing industry and 12 workplaces in the printing industry used 1,2-DCP. Detailed work processes in each industry included ultrasonic cleaning, manual cleaning, and mixing.
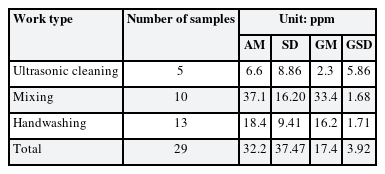
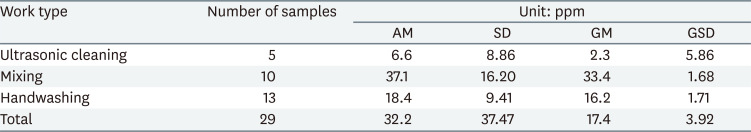
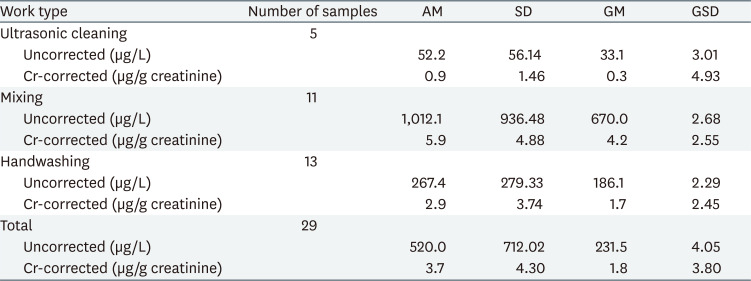
The exposure level of 1,2-DCP in the air was the highest in the mixing work type (geometric mean [GM] = 33.4 ppm, geometric standard deviation [GSD] = 1.68), and the GM concentration at handwashing was 16.2 ppm, and ultrasonic cleaning at 2.3 ppm. (Table 1).
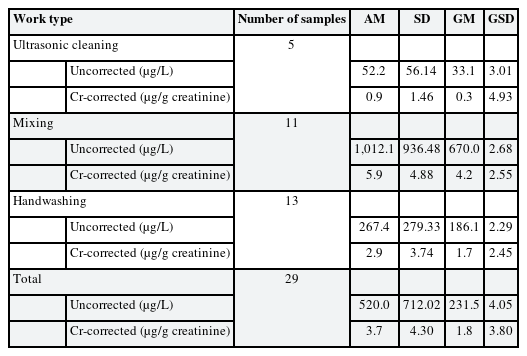
The concentrations of 1,2-DCP in urine (uncorrected and creatinine-corrected) by work type are summarized in Table 2. The distribution of 1,2-DCP concentration in urine are lognormal distribution (W = 0.984) and the GM (GSD) was 231.6 (4.05) µg/L.
Correlation between 1,2-DCP concentration in air and urine
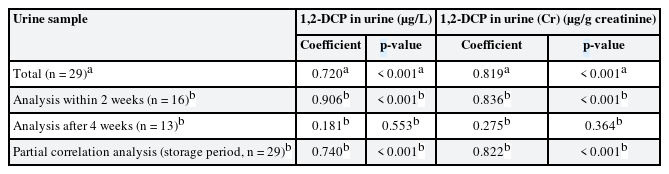
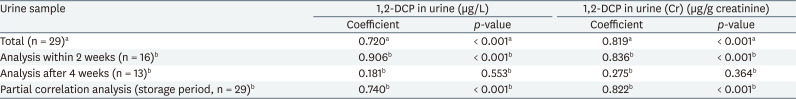
The correlation between 1,2-DCP concentrations in air and urine of workers, which were collected by personal sampling, was compared. The Pearson correlation coefficients of 1,2-DCP concentration in air (ppm) and urine (uncorrected and creatinine-corrected) were 0.720 and 0.819, respectively (Table 3).
For urine samples analyzed within 2 weeks (n = 16), the Spearman's rho of 1,2-DCP concentration in air and urine (uncorrected and creatinine-corrected) was 0.906 and 0.836, respectively. However, the correlation of urine samples analyzed after 4 weeks with the 1,2-DCP concentration in air was not significant (Table 3).
In the partial correlation analysis of 1,2-DCP concentration in air (ppm) vs. 1,2-DCP concentration in urine (creatinine-uncorrected, µg/L and creatinine-corrected, µg/g creatinine) with adjustment for duration of frozen storage, the partial correlation coefficients were 0.822 and 0.740, respectively (Table 3).
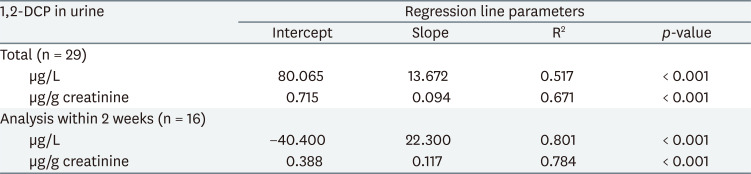
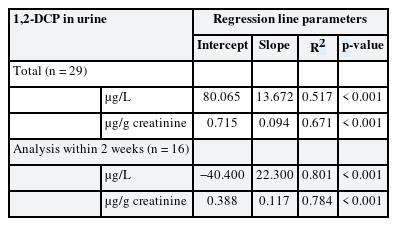
To estimate 1,2-DCP concentration in urine by 1,2-DCP concentration in air, simple regression analysis was performed. The results showed that the R2 values of 1,2-DCP concentration in urine and creatinine-corrected 1,2-DCP concentration in urine were 0.517 and 0.671, respectively (Table 4).
Simple regression analysis of 1,2-DCP in air and urine after work (total and samples analyzed within 2 weeks)
In addition, in simple regression analysis of the urinary concentration of the samples analyzed within 2 weeks, which showed the highest correlation, the R2 values of 1,2-DCP concentration in urine (uncorrected and creatinine-corrected, respectively) were 0.801 and 0.784, respectively (Table 4).
DISCUSSION
In this study, we aimed to investigate the correlation between 1,2-DCP concentration in air and urine to determine the appropriateness of measuring 1,2-DCP concentration in urine as a BEI for 1,2-DCP. Urine samples used for exposure assessment of biological monitoring need to be corrected by creatinine or urine specific gravity to adjust for dilution by seasonal or individual differences for each BEI. The results of the correlation analysis showed that R2 without correction was greater than that of the creatinine-corrected sample. Similar findings were reported by Kawai et al. [13] and this is possibly owing to the mechanism of transfer of unmetabolized 1,2-DCP into the urine. The underlying mechanism involves simple diffusion with no relation to the metabolism of creatinine or specific gravity-affecting substances in urine.
In agreement with the findings of Kawai et al. [13], this study also showed that a positive correlation and a dose-response relationship exists between the 1,2-DCP concentration in air and urine. In addition, the GM concentration of 1,2-DCP in the air of this study was 17.4 ppm (GSD = 3.92), higher than 7.1 ppm (GSD = 2.44) of Kawai et al.[13], so that this results can be used as a biological monitoring index for 1,2-DCP.
Based on the results of the regression analysis of this study, the BEI for the current exposure limit of 10 ppm can be presented as 1,2-DCP 0.2 mg/L in urine after completion of work. However, since 1,2-DCP has been designated as a special management substance since 2019, the level of exposure at the workplace may be lowered, so it is necessary to further study whether it can be used as a biological monitoring indicator even at low concentrations (less than 50% of exposure standards, below 5 ppm). In this study, the period of urine sample analysis was divided into “within 2 weeks” and “after 4 weeks” and the results of analysis within 2 weeks showed that the explanatory power for exposure-urinary concentration was significantly high.
There are several limitations to this study. First, the workers in the study were only employed by those in industries dealing with cleaning agents. In addition to workers using cleaning agents, it is necessary to study workers from various industries, such as machining processes using 1,2-DCP-containing tapping oil. Second, in this study and the previous study by Kawai et al. [13], 1,2-DCP in urine was analyzed by gas chromatography-mass spectrometry using the headspace method. Although this is a recognized method for analyzing urine samples, many typical analysis institutes do not have this equipment. Therefore, alternative analytical methods can be applied that have an advantage in terms of equipment cost. Third, this study did not compare the concentration of 1,2-DCP in urine before and after workers' work. There is no research data on the half-life of human excretion for 1,2-DCP. In animal research, the half-life of inhalation toxicity test for 3 hours and 80 ppm in rats suggests a half-life of 182 minutes in blood, 39 minutes in the lungs, and 57 minutes in the kidneys [14]. Considering this result, the half-life of urine excretion is within 3 hours, so it is judged that 1,2-DCP will not be detected in urine before work, but in order to select the correct urine collection time, the urine excretion half-life research is further needed.
CONCLUSIONS
As 1,2-DCP, a substitute cleaning agent for TCE, is an IARC group 1 carcinogen, there is an urgent need to manage its use in the future. Biological monitoring systems are one of the management measures. The results of this study can provide a basis for the establishment of such monitoring systems. Based on this study, 1,2-DCP concentration in urine without creatinine correction can be presented as an adequate biological exposure index for workers exposed to 1,2-DCP.
Notes
Funding: This work was supported by the Occupational Safety and Health Research Institute.
Competing Interests: The authors declare that they have no competing interest.
Author Contributions:
Conceptualization: Jeong KS, Ahn YS, Kim HS.
Data curation: Jeong KS, Kim HS, Park CS.
Formal analysis: Park CS, Jeong KS, Ahn YS.
Investigation: Jeong KS, Kim HS.
Writing - original draft: Park CS, Jeong KS, Kim HS.
Writing - review & editing: Jeong KS, Ahn YS, Kim HS.
Abbreviations
AM
arithmetic mean
BEI
biological exposure index
GM
geometric mean
GSD
geometric standard deviation
KBSI
Korea Basic Science Institute
IARC
International Agency for Research on Cancer
OSHA
Occupational Safety and Health Administration
SD
standard deviation
TCE
trichloroethylene
1,2-DCP
1,2-dichloropropane