The effects of exposure to lead, cadmium and mercury on follicle-stimulating hormone levels in men and postmenopausal women: data from the Second Korean National Environmental Health Survey (2012–2014)
Article information
Abstract
Background
Follicle-stimulating hormone (FSH), a gonadotropin secreted by the pituitary gland, is a representative secondary sex hormone and an important indicator of reproductive function. The effects of heavy metals such as lead, cadmium, and mercury on humans have been studied, but reports on their effects on sex hormone levels are lacking. Therefore, we investigated the relationship between heavy metal exposure and FSH levels in Korean men and postmenopausal women.
Methods
A total of 4,689 adults (2,763 men and 1,926 postmenopausal women aged 50 years or over) who participated in the Second Korean National Environmental Health Survey (2012–2014) were included. We compared differences in serum FSH levels by demographic characteristics using the t-test and analysis of variance. Multiple linear regression analysis was used to determine the relationship between the blood levels of lead and mercury and the urine cadmium level, and serum FSH levels.
Results
On multiple linear regression analysis, lead exposure was positively associated with serum FSH concentrations in postmenopausal women (β = 2.929, p = 0.019). However, we found no significant association between serum FSH concentration and blood lead and mercury levels, or urine cadmium level, in men.
Conclusions
This study suggests that lead exposure can affect the FSH level in postmenopausal women. Further studies are needed to evaluate the effects of low-dose long-term exposure to heavy metals on sex hormones.
BACKGROUND
Follicle-stimulating hormone (FSH), a gonadotropin secreted by the pituitary gland, stimulates spermatogenesis in males and ovarian follicle development in females. FSH is a representative secondary sex hormone and an important indicator of reproductive function [12]. Lead, cadmium, and mercury are common environmental heavy metals; many populations are exposed to these agents [3]. In recent decades, extensive toxicological studies have reported various adverse effects of the metals on humans, including reproductive toxicity [45].
Some studies have suggested that lead can increase the risk of spontaneous abortion through its potential teratogenic action [67]. Lead exposure was consistently associated with a reduced sperm count, poor sperm motility, and abnormal sperm morphology [8910]. Cadmium is a metalloestrogen stimulating the alpha and beta estrogen receptors and upregulating progesterone receptors [11]. Thus, cadmium may cause estrogen-dependent diseases such as breast and endometrial cancer, endometriosis, and spontaneous abortion [12]. Despite the well-known neurotoxicity of mercury, little is known about its potential effect on the human reproductive system. Some epidemiological studies described menstrual cycle abnormalities in women occupationally exposed to mercury [1314]. In men, methyl mercury levels in semen correlated with poor reproductive outcomes [15].
However, there are only a few studies about associations with sex hormones. A US study showed that serum FSH and luteinizing hormone (LH) levels increased as the blood lead level rose in both pre- and post-menopausal women and in those who had both ovaries removed [16]. Also, other US data showed a positive association between lead and testosterone levels in males, and cadmium and FSH levels in perimenopausal women [1718]. In China, a recent study found positive associations between lead and testosterone levels in men and between lead and FSH and LH levels in postmenopausal women [19].
Although a few studies have evaluated the effect of heavy metals on FSH in human, the evidence of the effect on FSH is limited. In this study, we evaluated the relationship between exposure to lead, cadmium, and mercury and FSH in Korean men and postmenopausal women.
METHODS
Study participants
We used data from the Second Korea National Environmental Health Survey (KoNEHS) conducted by the National Institute of Environmental Research from 2012 to 2014; the survey involved stratified sampling of the national population based on the 2010 housing census. The survey included 6,478 subjects aged > 19 years from 400 districts selected based on the population distribution. Data were collected via personal interview, physical examination, and laboratory tests. Men (n = 2,774) and postmenopausal women (n = 2,027) were selected. Women under 50 years of age who described themselves as postmenopausal (n = 91) were excluded. Subjects for whom data were missing (n = 21) were also excluded. Finally, 2,763 men and 1,926 women were included in the analysis.
Variables
Age, sex, menopausal status, body mass index (BMI), smoking status, and alcohol consumption were included as variables. All subjects were men or postmenopausal women. BMI was divided into 4 groups: underweight (< 18.50 kg/m2), healthy weight (18.50–24.99 kg/m2), overweight (25.00–29.99 kg/m2), and obese (≥ 30.00 kg/m2). Smoking status was based on questionnaire data: smoker or current non-smoker. Alcohol consumption was categorized as yes or no.
Blood lead and mercury, urine cadmium levels and serum FSH
Blood lead and mercury, urine cadmium levels and serum FSH were analyzed by the following methods in KoNEHS [2021]. Blood and spot urine specimens were transferred to the laboratory in an ice box and stored at −20°C prior to analysis. Blood lead and urine cadmium levels were assayed with the aid of a Graphite Furnace-Atomic Absorption Spectrometer. Blood mercury was analyzed using a gold amalgamation technique. The concentrations of all metals were derived with the aid of standard calibration curves. The limits of detection (LODs) were 0.30 for blood lead, 0.05 for urinary cadmium, and 0.1 µg/L for blood mercury. Measurements below the LOD were recorded as the LOD divided by the square root of 2. Urine cadmium concentration was adjusted by reference to urine creatinine concentration. Serum FSH levels were measured using a chemiluminescence immunoassay (CLIA; ADVIA Centaur XP; Siemens, Tarrytown, NY, USA).
Statistical analyses
Geometrical means with 95% confidence interval (CI) of serum FSH concentrations were calculated by reference to demographic factors; we also derived the medians and geometric means with 95% CI of blood lead and mercury, and urine cadmium, levels. As the distributions of all heavy metals and FSH in men were positively skewed, data of these factors were log-transformed prior to analyses. But, FSH in postmenopausal women showed normal distribution, so it was not log-transformed. Univariate analyses by demographic characteristics were performed employing the t-test and analysis of variance. Multiple linear regression analysis was used to determine the relationship between lead, cadmium and mercury exposure, and serum FSH concentrations; after adjusting for age, BMI, smoking status, and alcohol consumption of men and postmenopausal women separately. SPSS ver. 25 for Windows (IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
Ethics statement
This study was approved by the Institutional Review Board (IRB) of Haeundae Paik Hospital (IRB No. 2019-04-008).
RESULTS
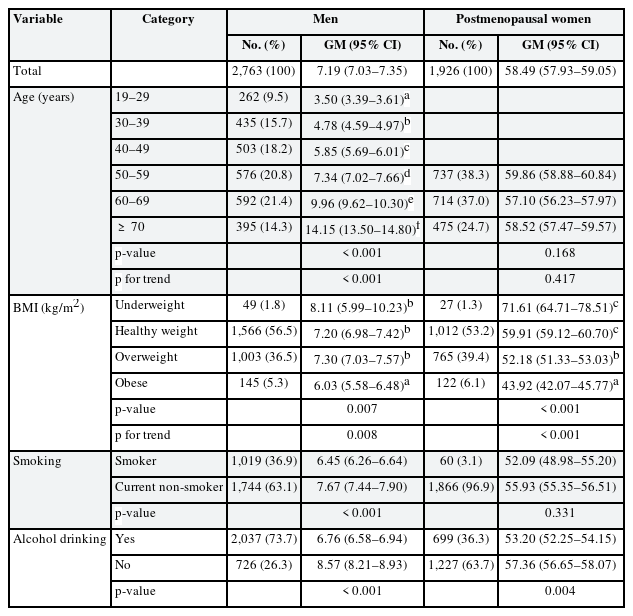
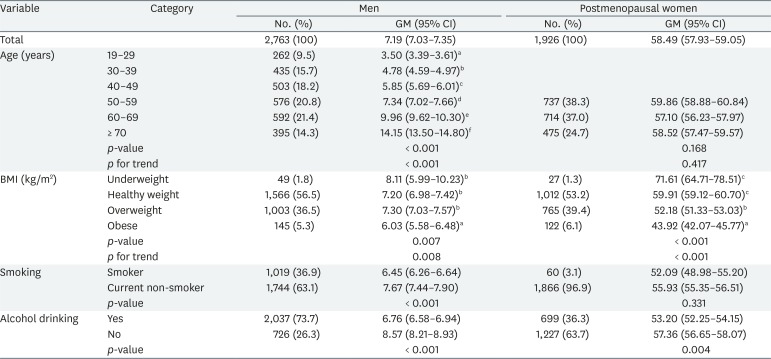
The geometric means of serum FSH concentrations by demographic variables are shown in Table 1. Total geometric means were 7.19 and 58.49 mIU/mL in men and postmenopausal women, respectively. In men, the geometric mean tended to increase with age (p for trend < 0.001) and decrease with increasing BMI (p for trend = 0.008). In postmenopausal women, the geometric mean tended to decrease with increasing BMI (p for trend < 0.001), as in men, but was not affected by age. In men, smoking (p < 0.001) and alcohol consumption (p < 0.001) significantly affected FSH levels; in women, only alcohol consumption was significant (p = 0.004).
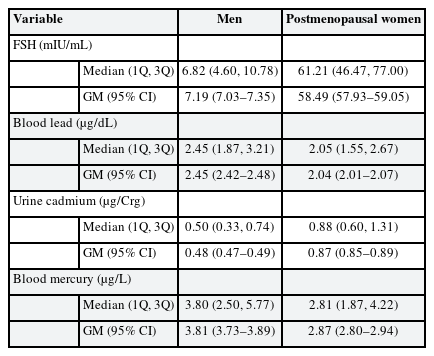
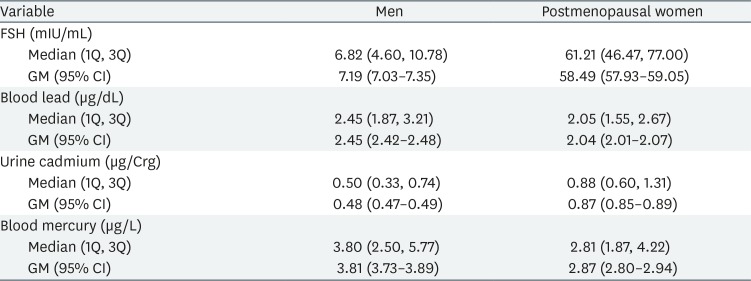
The distributions of serum FSH concentrations, and blood lead and mercury, and urine cadmium levels, are listed in Table 2; we show medians, first and third quartile data, and geometric means with 95% CI.
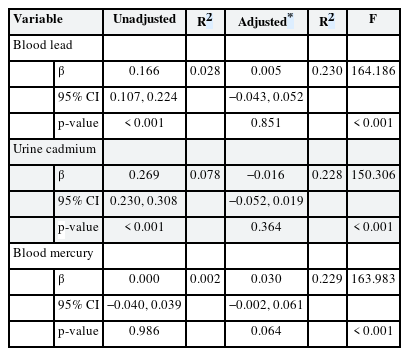
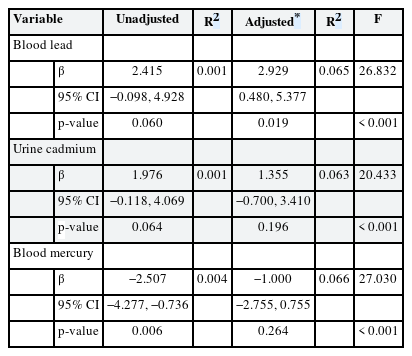
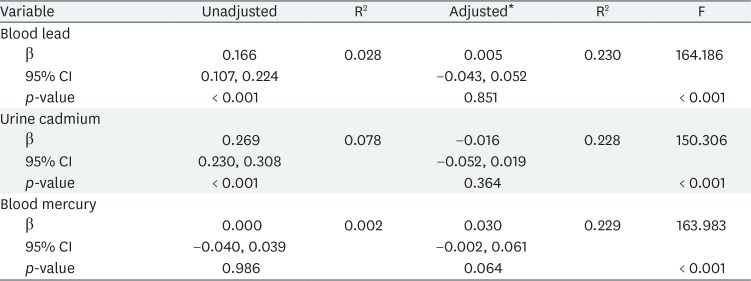
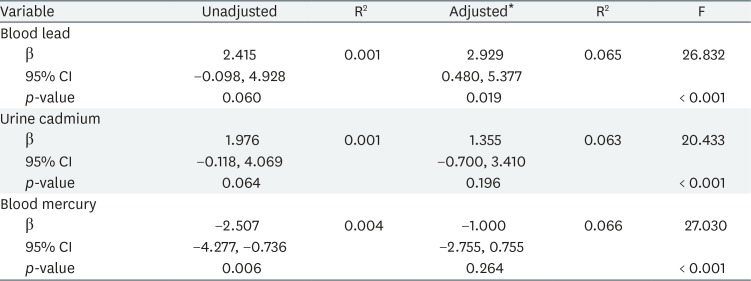
Tables 3 and 4 list the results of multiple linear regression analyses in men and women, respectively; we show the adjusted and unadjusted regression coefficients between lead, cadmium and mercury levels, and FSH concentrations. In women, FSH was significantly associated with blood lead level in the adjusted model (β = 2.929, p = 0.019). However, in men, no significant association between heavy metal levels and FSH concentration was evident after adjustment.
DISCUSSION
We explored the relationship between lead, cadmium and mercury exposure, and FSH levels. Our results suggest that lead exposure can affect serum FSH concentration in postmenopausal women. A few epidemiological studies have explored the association between heavy metal and FSH levels. Our results are consistent with those of earlier works from the USA and China [1619]. In China, a recent study found statistically significant positive associations between the blood lead level and FSH concentration in postmenopausal women, but the significance was marginal in men [19]. We obtained similar data from postmenopausal women, but found no significant association in men. A few studies on the relationship between cadmium and FSH levels have been performed in the USA and Italy; positive associations were reported in perimenopausal women and male workers [1822]. However, we found that urine cadmium and blood mercury levels were not associated with FSH concentrations in Korean adults.
In Table 1, we analyzed serum FSH concentrations by demographic variables. As age increased, FSH levels tended to increase in men, which can be explained by the compensatory increase of FSH due to decreasing function of primary reproductive organs [23]. FSH levels fell with increasing BMI in both men and women, attributable to negative feedback by sex hormones secreted by adipose tissue [24]. A previous study suggested that cigarette smoking increased the serum levels of several sex hormones including testosterone in men; this reduces FSH levels via negative feedback [25]. Alcohol triggers hypothalamic-pituitary inhibition, reducing FSH levels, consistent with our results [26].
We found a positive association between blood lead and FSH levels in postmenopausal women. In several studies in mice and rats, lead accumulated in the ovaries and reduced the number of follicles and the extent of the corpus luteum [272829]. Paksy et al. [30] found that lead accumulated in human ovarian follicular fluid, and decreased progesterone production by granulosa cells in vitro. As mentioned above, lead triggers a compensatory increase in FSH by disrupting the function of the ovary, the primary female reproductive organ [23]. Also, lead increases FSH concentrations by acting on the hypothalamus and pituitary gland [31], either directly or via interactions with calcium or cellular proteins [3233]. Lead stimulates neurotransmitter secretion by inhibiting calcium flow through the calcium channel [34]. Also, lead binds to and activates calmodulin, triggering the signaling cascade that controls the pulsatile release of gonadotropin-releasing hormone (GnRH) from hypothalamic cells [3536]. These actions are possible because lead can cross the blood-brain barrier to disturb directly the hypothalamic-pituitary axis [3738]. In rats, long-term low-dose lead exposure significantly increased the level of mRNA encoding GnRH [39]. And, lead can increases FSH levels indirectly by elevating homocysteine concentrations [40]. Homocysteine serves as an agonist of both N-methyl-D-aspartate (NMDA) and γ-aminobutyric acid (GABA) [4142]. NMDA can upregulate FSH secretion and GABA is an important factor of the regulation of GnRH secretion [4344].
Endocrine disruptors are effective at low concentrations; even small changes in hormone concentrations can trigger biological effects [45]. Lead exposure may also have adverse effects with increase in FSH on men and postmenopausal women. Increased FSH levels can contribute to osteoclast formation and increased bone resorption [4647]. Since FSH has been shown to directly stimulate bone resorption both in vitro and in vivo, serum FSH levels are widely recognized as predictors of bone loss [48]. Previous studies found that elevation in FSH was an independent risk factor for bone loss in postmenopausal women and, indeed, aided early osteoporosis diagnosis [49]. In men, a recent study found a longitudinal inverse relationship between higher FSH levels and lower bone mineral density; men with higher levels of FSH lost more bone over time [50].
The principal strength of our present study is that we are the first to assess the relationship between exposure to lead, cadmium and mercury, and FSH levels, in a Korean population. We add to prior knowledge of the effects of heavy metal exposure on FSH concentrations. However, our work had certain limitations. First, we lacked data on the long-term effect of heavy metal exposure on FSH levels; the KoNEHS is a cross-sectional observational study. Second, in the KoNEHS, there was no information on the confounding factors including history of gynecological disease, hormonal replacement therapy. Therefore, we could not consider these factors to derive outcomes in the analyses. These confounding factors could affect our results, thus, our results should be interpreted with caution. Third, other sex hormones, such as LH, estrogen, and testosterone, were not measured in the KoNEHS. Thus, in this study, it was limited to show the association with overall sex hormones because we could only evaluate the association with FSH. Additional studies are needed to research the association with other sex hormones.
The health risks posed by heavy metals including lead, cadmium, and mercury constitute a public health concern, regardless of whether exposure is occupational in nature. FSH is a representative secondary sex hormone; we evaluated the effects of heavy metals on reproductive function by examining their effects on FSH levels. Future studies should address the limitations of our present work.
CONCLUSIONS
This study suggests that lead exposure can affect the FSH level in postmenopausal women. Further studies are needed to evaluate the effect of low-dose long-term exposure to heavy metals on the levels of various sex hormones.
Acknowledgements
This study used data from the Second Korean National Environmental Health Survey (2012–2014), which was conducted by National Institute of Environmental Research. The authors gratefully acknowledge their effort.
Notes
Competing interests: The authors declare that they have no competing interests.
Availability of data and materials: The datasets analyzed during the current study are available on request at the National Institute of Environmental Research, Environmental Health Research Department, http://www.nier.go.kr/NIER/kor/openapi/getAsub.do?menuNo=14010.
Author Contributions:
Data curation: Kim DH.
Formal analysis: Lee TW, Ryu JY.
Investigation: Ryu JY.
Writing - original draft: Lee TW, Ryu JY.
Abbreviations
BMI
body mass index
CLIA
chemiluminescence immunoassay
CI
confidence interval
FSH
follicle-stimulating hormone
GABA
γ-aminobutyric acid
GnRH
gonadotropin-releasing hormone
GM
geometric mean
KoNEHS
Korean National Environmental Health Survey
LH
luteinizing hormone
LOD
limit of detection
NMDA
N-methyl-D-aspartate
1Q
1st quartile
3Q
3rd quartile