Collective exposure to lead from an approved natural product-derived drug in Korea
Article information
Abstract
Background
In Asian countries, including Korea, lead poisoning caused by traditional herbal medicines is often observed in the clinic. However, there have been no reports thus far of lead poisoning caused by drugs that were approved by the Korea Food and Drug Administration (KFDA). Here, we describe seven patients who ingested a problematic natural product-derived drug (NPD).
Case presentation
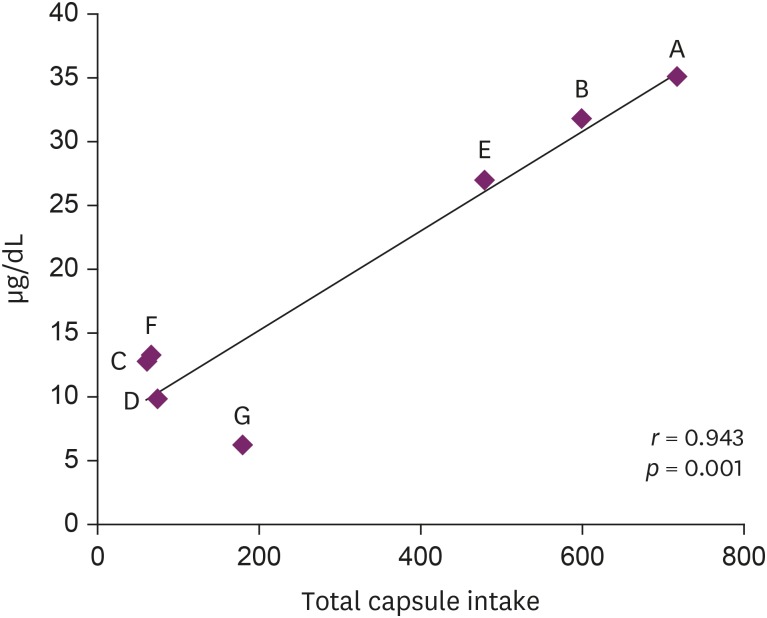
In July 2018, seven patients visited a university hospital after ingesting an NPD, S. capsules. These patients complained of various symptoms, and their blood lead levels (BLLs) were elevated relative to those of the general population (arithmetic mean: 19.5 ± 11.6 µg/dL, range: 6.28–35.25 µg/dL). The total doses and BLLs were directly proportional to each other among the patients (r = 0.943, p = 0.001). After the patients discontinued drug intake, their BLLs decreased gradually. The capsule was confirmed to contain lead above the standard value (arithmetic mean: 2,547 ± 1,821.9 ppm).
Conclusion
This incident highlights the need to strengthen standards for the management of NPD ingredients in Korea. NPDs are more likely to be contaminated than other drugs. Thorough management by the KFDA is essential to prevent a recurrence. Moreover, systematic health care is needed for many patients who have taken problematic NPDs.
BACKGROUND
Lead is a representative heavy metal that causes acute or chronic adverse health effects in multiple organ systems [1]. Lead poisoning can be caused by occupational and environmental exposures. Lead poisoning due to occupational exposure has been greatly reduced in recent years, but environmental exposure is a persistent problem [234]. Blood lead levels (BLLs) in Korean lead workers were 3.53 µg/dL in 2011 [2]. According to the Korean National Health and Nutrition Examination Survey (KNHANES), the BLL in the Korean general population was 1.70 µg/dL in 2016 [5]. The National Institute for Occupational Safety and Health (NIOSH) recommended that the BLL for adults be lowered to < 10 µg/dL [6].
In Korea, natural product-derived drugs (NPDs) are derived from natural products but are not formulated by the extraction and purification of a specific ingredient. Thus, NPDs are used as medicines or as raw materials for medicines following simple processes such as drying, cutting, or powdering natural products. The Korea Food and Drug Administration (KFDA) has licensed these NPDs as over-the-counter (OTC) drugs, in accordance with the legitimate reporting procedure. NPDs are sold through pharmacies without a doctor's prescription. In June 2018, the KFDA reported that lead in excess of the standard value was detected in S. capsules. S. capsule is T. capsule's copy drug sold about 2 million dollars in Korea. Cicadidae Periostracum, Hirudo in S. capsule reported to be contaminated with lead. The estimated amount of contaminated material was 468 kg and 324 kg, respectively. After the press release, group of patients taking these capsules knows about KFDA's report from pharmacy, they visited our university hospital and underwent blood lead tests.
Notably, in Asian countries, including Korea, lead poisoning caused by traditional herbal medicines is often observed in the clinic [7]. However, lead poisoning has thus far not been reported by a group of patients who were taking herbal medicines approved and administered by the KFDA. In the present case series, we describe seven patients who ingested the problematic NPD.
CASE PRESENTATION
Drug information
Product: S. capsules (Fig. 1).

(A) Case drugs: S. capsules (manufacturing number 18002). (B) Metallic components are observed in (a) the case drugs (arrows), but not in (b) the control drugs (usual common cold drugs include acetaminophen).
Company: I. Pharmaceutical Co. Ltd., Yongin-si, Republic of Korea.
Classification: OTC drug, Korea Good Manufacturing Practice (KGMP)-approved natural product medicine.
Route: Oral (solid).
Drug effect
1. Improves blood circulation in patients recovering from cerebral thrombosis.
2. Relief from angina symptoms.
Components, included in 1 capsule
1. Cicadidae Periostracum 68.75 mg
2. D-Borneol 13.75 mg
3. Eupolyphaga 88.75 mg
4. Ginseng 75 mg
5. Hirudo 103.75 mg
6. Peony Root 82.5 mg
7. Scolopendrae Corpus 43.75 mg
8. Scorpion 78.75 mg
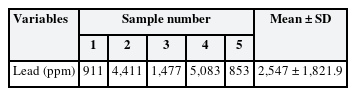
S.capsule's components are described in Korea Pharmaceutical Information [8]. The results of a lead content analysis are described in Table 1. Five samples were analyzed, and the arithmetic average of the lead content was 2,547 ± 1,821.9 ppm (Table 1).
Lead analysis
For blood lead analysis, 0.1 mL of whole blood was diluted with 0.8 mL of 1% Triton X-100 and the diluted solution was analyzed for BLL using graphite furnace atomic absorption spectrometry (AA-7000; Shimadzu, Kyoto, Japan). The standard addition method was used to prepare the standard curve. We randomly picked 5 capsules in manufacturing number 18002. An analysis of the capsules for lead done using an inductively coupled plasma optical emission spectrometer (ICP-OES, Avio 500; Perkin-Elmer, Norwalk, MA, USA).
Patient information
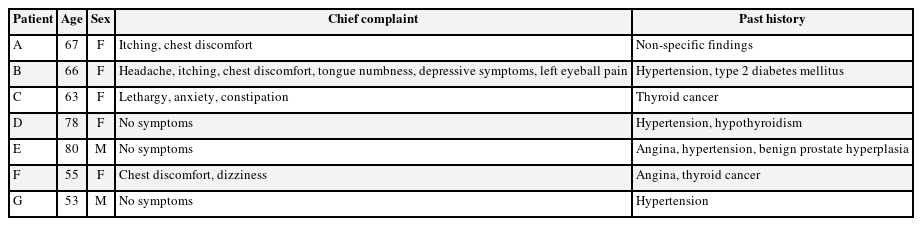
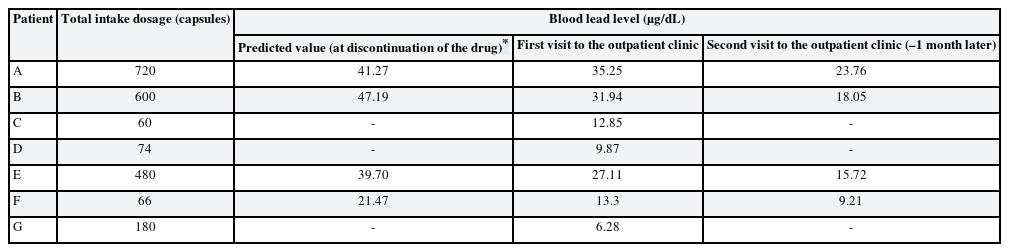
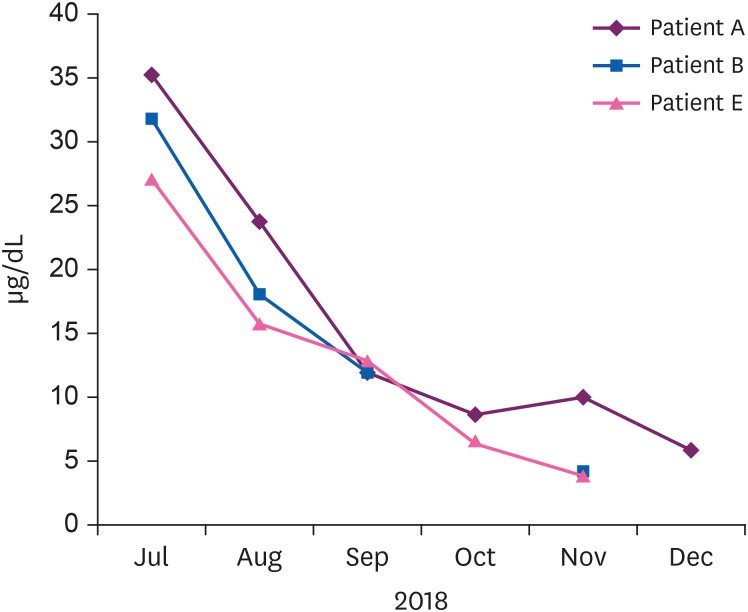
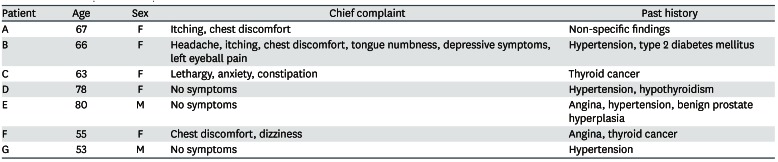
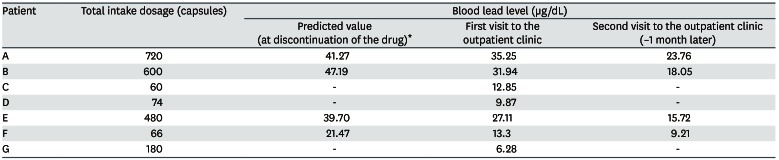
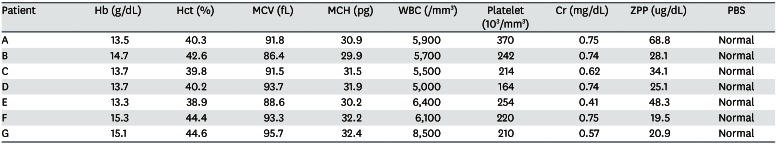
Eight patients visited the hospital and we reported seven cases who agreed to this study (Table 2). Each subject provided written, informed consent before participating. Of 7 cases, we present the details of 3 with a high BLL and show summary results for the other cases in Tables 2, 3, 4. Seven patients complained of various clinical symptoms and showed elevated BLLs of 6.28–35.25 µg/dL, which were higher than that in the general population. The total doses and BLLs were directly proportional to each other (Fig. 2). After the patients discontinued taking the drug, their BLLs decreased gradually (Fig. 3). To calculate predicted values when drug intake was discontinued, we used the difference between the first and second BLLs, as well as the days elapsed between measurements. The predicted BLL values were 41.27 µg/dL in patient A and 47.19 µg/dL in patient B (Table 3).
Ethics statement
Written informed consents were obtained from the patient for publication of this case report and any accompanying data.
Patient A
A 66-year-old woman.
Chief complaints
1) Headache; 2) Itching sensation; 3) Chest discomfort; 4) Tongue numbness; 5) Depressive symptoms; 6) Left eyeball pain.
Past medical history and family disease
1) Hypertension medication for 10 years; 2) Diabetes mellitus medication for 15 years.
Occupational and environmental factors
No specific findings.
History of present illness
The patient ingested 720 capsules over 6 months. She visited our hospital for a lead test upon learning from a pharmacist that lead had been found in the capsules.
Abnormal physical and laboratory results
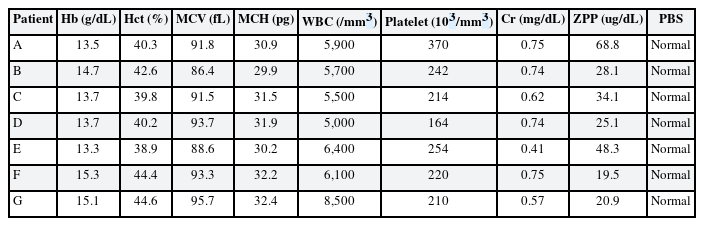
Elevated blood lead and zinc protoporphyrin (ZPP) levels were observed at her first visit to our hospital. The initial ZPP level was 68.8 µg/dL, which was higher than the reference value (< 35 µg/dL). Her initial BLL was 35.25 µg/dL; it returned to the normal range (8.64 µg/dL) after 120 days. Complete blood cell count (CBC), peripheral blood smear (PBS), and renal function test results were normal.
Assessment of lead dose
1.413 mg/capsule × 720 capsules = 1,017.78 mg
Treatment
Conservative treatment was performed without chelation treatment [9].
1) Prohibition of S. capsule intake; 2) Multivitamin, 1 capsule per day; 3) Green tea, 1,000 mL per day; 4) Garlic, 20–40 g per day.
Progression
The patient's tongue tingling and systemic symptoms improved within 4 months, but her anxiety, depression, and weakness persisted. Brain magnetic resonance imaging performed at our Neurology Department was normal except for mild chronic small vessel ischemic disease. Six months after discontinuing S. capsule intake, the patient remains under treatment for depression and anxiety in the Psychiatry Department, and for dysesthesia in the Neurology Department.
Patient B
A 63-year-old woman.
Chief complaint
1) Anxiety; 2) Constipation.
Past medical history and family disease
Thyroidectomy due to papillary thyroid cancer.
Occupational and environmental factors
No specific findings.
History of present illness
The patient ingested 600 capsules over 6 months. She visited our hospital for a lead test upon learning from a pharmacist that lead had been found in the capsules. She exhibited great concern with respect to lead poisoning at her first visit.
Abnormal physical and laboratory results
Her systolic blood pressure and diastolic blood pressure were 195 mmHg and 96 mmHg, respectively. An elevated BLL and elevated lipid profile were observed at her first visit to our hospital. Drug treatment was administered for hypertension and hyperlipidemia. CBC, PBS, and renal function test results were normal. The initial ZPP level was 28.1 µg/dL, which was normal. Her initial BLL was 31.94 µg/dL; it returned to the normal range (4.12 µg/dL) after 120 days.
Assessment of lead dose
1.413 mg/capsule × 600 capsules = 848.15 mg
Treatment
Identical to that for Patient A.
Progression
The patient's constipation and anxiety were improved. After her BLL reached a normal level, follow-up was discontinued.
Patient E
A 55-year-old woman.
Chief complaint
1) Chest discomfort; 2) Dizziness; 3) Dysesthesia in the upper and lower limbs; 4) Skin rash.
Past medical history and family disease
1) Variant angina medication for 2 years; 2) Left lobectomy due to thyroid cancer.
Occupational and environmental factors
No specific findings.
Abnormal physical and laboratory results
An elevated BLL and elevated ZPP level were observed at her first visit to our hospital. The initial ZPP level was 48.3 µg/dL, which was higher than the reference value. Her initial BLL was 27.11 µg/dL; it returned to the normal range (9.21 µg/dL) after 90 days. CBC, PBS, and renal function test results were normal.
Assessment of lead dose
1.413 mg/capsule × 480 capsules = 678.52 mg
Treatment
Identical to that for Patient A.
Progression
Chest tightness and palpitations persisted; thus, coronary angiography was performed. No other cause for the patient's chest symptoms was found. Seven months after discontinuing S. capsule intake, the patient remains under treatment for chest discomfort in the Cardiology Department. A persistent skin rash and pain are present in the upper and lower limbs, and lethargy is present throughout; the patient continues to show improvement and exacerbation in an alternating manner. Symptomatic treatment is performed in our Dermatology and Neurology Departments.
DISCUSSION AND CONCLUSION
The arithmetic mean BLL at the first visit after discontinuing the medication was 19.5 ± 11.6 µg/dL higher than that of the general population; the total dose taken and BLL were proportional. An analysis of the lead content of the capsules revealed that the arithmetic average was 2,547 ± 1,821.9 ppm, which was much higher than the Korean Pharmacopoeia standard of 5 ppm (range: 853–5,083 ppm). KFDA investigations confirmed that the ingredients of Cicadidae Periostracum and Hirudo contained lead above the standard value.
Kosnett et al. [10] suggested that chelation therapy is needed when a patient's BLL is > 50 µg/dL with significant symptoms or signs of lead toxicity. In addition, Centers for Disease Control and Prevention (CDC) management guidelines for children with elevated blood levels suggest that chelation therapy is needed when the BLL is > 45 µg/dL [11]. The BLLs were reduced in all patients without implementing chelation therapy. There was no evidence of damage to target organs such as the kidneys and hematopoietic system based on the combined results of interviews and tests. Most patients complained of chest discomfort because of the use of S. capsule as a drug for angina pectoris. Among patients with high BLLs, those with neurological symptoms (e.g., headache and abnormal sensations) were symptomatically treated in the Neurology Department. Patients with depression and anxiety were treated in the Psychiatry Department. There were no other symptoms of increased blood lead, as determined by interviews. There was a tendency for blood lead to decrease after drug withdrawal. However, last follow-up observation was not still normal levels in 3 patients and continuous follow-up was needed. Overall, blood lead was judged to have increased due to the ingestion of S. capsules.
In Asian countries, including Korea, traditional medicine has become remarkably advanced. In patients with a chronic illness, herbal medicine is often used as a substitute therapy if conventional medication shows a weak therapeutic effect [12]. Many patients believe that herbal medicines are natural and therefore safe. However, the contamination or adulteration of herbal medicines is possible and can cause harm [13]. Because of the many uses and uncontrolled distribution processes of herbal medicines, they often exhibit high concentrations of heavy metals [141516]. The World Health Organization reported that inappropriate use of herbal medicine can have negative or dangerous effects; therefore, efficacy and safety should be assessed for these medicines [17]. Kim et al. [7] published a review that described adverse events associated with heavy metal contamination of traditional Chinese medicines in Korea. In total, ten publications were included in the review. One study found 45 cases of lead poisoning in Korea during the period from 1973 to 2002. Another 9 Korean case reports or case series were identified (22 cases in total) that involved heavy metal poisoning caused by traditional Chinese medicines. Additional unreported cases are likely. Among all available reports, none have described increased BLLs in a group of patients taking KFDA-approved drugs.
In the present report, the BLLs were lower than in a previous report [7]. The highest BLL in the present report was 35.25 µg/dL, whereas it exceeded 40 µg/dL in the previous report. However, the half-life was calculated as 25.4–43.9 days in three patients in the present report; therefore, the predicted BLL was > 40 µg/dL in patients A and B. The CDC has recommended that the BLL for adults be lowered below 10 µg/dL [6]. Furthermore, recent studies have raised concerns of lead toxicity at low BLLs [1819]. According to Korea Health Statistics 2016 [5], the lead concentration in Korean adults was 1.70 µg/dL. Therefore, the lead concentrations in the present report were extremely high. Here, there were no specific findings in clinical tests other than elevated BLLs. Basophilic stippling is known to be seen in PBS results of lead poisoning patients [20] but this was not seen in these cases, which may be due to relatively low blood lead concentration. Neuropsychological problems such as tiredness, somnolence, moodiness, and reduced interest in leisure activities initially appear at BLLs of 20–69 µg/dL; headache, gastrointestinal symptoms, peripheral neuropathy, nephropathy, and anemia appear at BLLs of 70–100 µg/dL; and intracranial hypertension and delirium begin to manifest when BLLs exceed 100 µg/dL, along with wrist drop, foot drop, and encephalopathy [21]. In the present case series, patients with relatively high BLLs complained of a headache and the neuropsychological symptoms noted above. If the problematic NPD was ingested for an extended period, a serious situation may have occurred. Medicines containing herbal medicine ingredients tend to be contaminated because they use unprocessed raw materials, whereas conventional medicines do not. Also, herbal medicines tend to be contaminated in manufacturing process [22]. In this case report, it has been confirmed that it is manufactured at an inappropriate place, production records were manipulated. It is difficult to determine whether raw materials are exposed to contaminants such as heavy metals. Furthermore, NPDs are sold through pharmacies without a doctor's prescription; therefore, it can be difficult to determine who might have ingested such drugs. In this case, the author formally reported to the KFDA about our patients. In this case, the author has officially reported to the KFDA about our patients. However, it is thought that many users of this drug may not be aware of the situation accurately. Production quantity per each serial number is about 2,000 boxes (360 capsules) and, the KFDA found 8 serial numbers that had problems. If patients bought one boxed product per person, there could be up to 16,000 victims. More rigorous management systems for NPDs in the future are essential.
In conclusion, efforts should be made to improve the approval and management system of the KFDA to ensure safety and prevent a recurrence of this situation. New, strengthened management standards for heavy metals should be established with respect to herbal medicine ingredients. In addition, aggressive health impact assessments and management should be performed for all patients taking S. capsules.
Acknowledgements
We thank all the members of the Health Promotion Center at Chonnam National University Hwasun Hospital. We also thank the patients for their voluntary participation.
Notes
Competing interests: The authors declare that they have no competing interests.
Author Contributions:
Conceptualization: Lim DY, Park WJ.
Data curation: Kang WY, Cho S, Lee BC.
Formal analysis: Lim DY, Kim S, Park WJ.
Investigation: Lim DY, Ahn JS, Kim S, Park WJ.
Writing - original draft: Lim DY.
Writing - review & editing: Moon JD, Park WJ.
Abbreviations
BLL
Blood lead level
CBC
Complete blood cell count
CDC
Centers for Disease Control and Prevention
KFDA
The Korea Food and Drug Administration
KGMP
Korea Good Manufacturing Practice
KNHANES
Korean National Health and Nutrition Examination Survey
NIOSH
National Institute for Occupational Safety and Health
NPD
natural product-derived drug
OTC
over-the-counter
PBS
peripheral blood smear
ZPP
zinc protoporphyrin