Does formaldehyde have a causal association with nasopharyngeal cancer and leukaemia?
Article information
Abstract
Background
The South Korean criteria for occupational diseases were amended in July 2013. These criteria included formaldehyde as a newly defined occupational carcinogen, based on cases of “leukemia or nasopharyngeal cancer caused by formaldehyde exposure”. This inclusion was based on the Internal Agency for Research on Cancer classification, which classified formaldehyde as definite human carcinogen for nasopharyngeal cancer in 2004 and leukemia in 2012.
Methods
We reviewed reports regarding the causal relationship between occupational exposure to formaldehyde in Korea and the development of these cancers, in order to determine whether these cases were work-related.
Results
Previous reports regarding excess mortality from nasopharyngeal cancer caused by formaldehyde exposure seemed to be influenced by excess mortality from a single plant. The recent meta-risk for nasopharyngeal cancer was significantly increased in case-control studies, but was null for cohort studies (excluding unexplained clusters of nasopharyngeal cancers). A recent analysis of the largest industrial cohort revealed elevated risks of both leukemia and Hodgkin lymphoma at the peak formaldehyde exposure, and both cancers exhibited significant dose-response relationships. A nested case-control study of embalmers revealed that mortality from myeloid leukemia increased significantly with increasing numbers of embalms and with increasing formaldehyde exposure. The recent meta-risks for all leukemia and myeloid leukemia increased significantly. In South Korea, a few cases were considered occupational cancers as a result of mixed exposures to various chemicals (e.g., benzene), although no cases were compensated for formaldehyde exposure. The peak formaldehyde exposure levels in Korea were 2.70–14.8 ppm in a small number of specialized studies, which considered anatomy students, endoscopy employees who handled biopsy specimens, and manufacturing workers who were exposed to high temperatures.
Conclusion
Additional evidence is needed to confirm the relationship between formaldehyde exposure and nasopharyngeal cancer. All lymphohematopoietic malignancies, including leukemia, should be considered in cases with occupational formaldehyde exposure.
Background
The South Korean Schedule for the Enforcement Decree of the Industrial Accident Compensation Act was amended in July 2013 to provide specific criteria for the recognition of occupational diseases, including occupational cancer. This amendment increased the official recognition from 9 cancers and 9 carcinogens to 21 cancers and 23 carcinogens. For example, the amendment formally recognized “leukemia and nasopharyngeal cancer [NPC] caused by formaldehyde [formaldehyde] exposure” [1, 2]. However, there are few detailed scientific reviews that have considered the relationship between formaldehyde exposure and leukemia in Korea.
The International Agency for Research on Cancer (IARC) officially classified formaldehyde as a “definite human carcinogen” for NPC in 2004 and for leukemia in 2012 [3, 4]. Other authorities have also acknowledged the possibly carcinogenic role of formaldehyde, including the American Environmental Protection Agency (EPA), the European Union Occupational Disease Classification, Labelling and Packaging of Substances and Mixtures (EU CLP) guidelines, and the American Conference of Governmental Industrial Hygienists (ACGIH) [5–7]. However, the 2010 amendment of the International Labor Organization (ILO) guidelines did not reach a consensus regarding whether occupational formaldehyde exposure was directly linked to NPC or leukemia [8]. Nevertheless, formaldehyde-related cancers are included in the lists of recognized occupational diseases in France, Denmark, Taiwan, and Malaysia [9–11].
Given the international trend towards recognizing formaldehyde-related occupational disease, and the absence of Korean reviews, we reviewed epidemiological studies and other evidence from Korea. We also present points for consideration during the process of determining whether formaldehyde-related NPC and leukemia should be considered work-related.
Methods
We initially reviewed various scientific papers, including many epidemiological studies, regarding the causal relationship between formaldehyde exposure and cancer. Second, we reviewed various scientific papers, industrial reports, occupational exposure level reports, population data, and task force reports regarding exposure in Korean. Third, we reviewed various reports and epidemiological studies (including cohort studies, case-control studies, meta-analyses, reviews, and experimental studies) regarding the carcinogenicity of formaldehyde. Fourth, we investigate the national regulations regarding officially recognized occupational diseases and the international classifications for carcinogenicity. Finally, we considered the issues that could influence or determine the causal relationship between occupational exposure and cancer.
Results
Use and exposure in South Korea
Formaldehyde is mainly used in the production of various resins, although it is also used extensively as an intermediate during the manufacture of various industrial chemicals and directly as an aqueous disinfectant [3, 4]. The highest average exposures (2–5 ppm; 2.5–6.1 mg/m3) were measured during furniture and floor varnishing, textile finishing, fur treatment, in the garment industry, and in certain jobs at manufactured board mills and foundries. Short-term exposures to high levels (≥3 ppm; ≥3.7 mg/m3) have been reported for embalmers and pathologists [3].
In South Korea, employees who are exposed to formaldehyde have regular mandatory health examinations, which are mandatory for workers who are exposed to workplace hazards. Approximately 18,000 employees had formaldehyde-related health examinations during 2008, which accounted for 2.07% of all specific health examinations. Almost all specific health examinations were performed for people who worked in the manufacturing industry or in health and social work activities (Table 1).
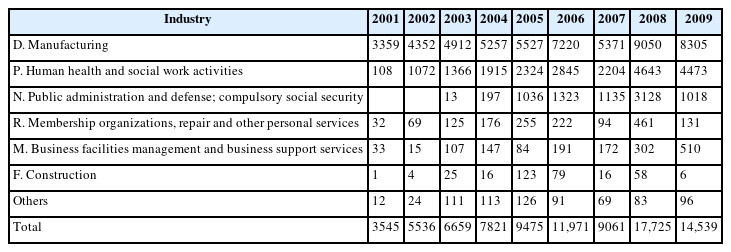
Number of workers examined special health check for formaldehyde by types of industries and years in Korea, 2001–2010
Table 2 shows the formaldehyde exposure levels using a threshold limit value-time weighted average (TLV-TWA), based on national data regarding working environment measurements from 2002 to 2010. The highest number of samples was observed in industries that manufactured chemicals and chemical products, which was followed by the manufacture of motor vehicles, trailers and semitrailers, and non-furniture wood and cork products [12].
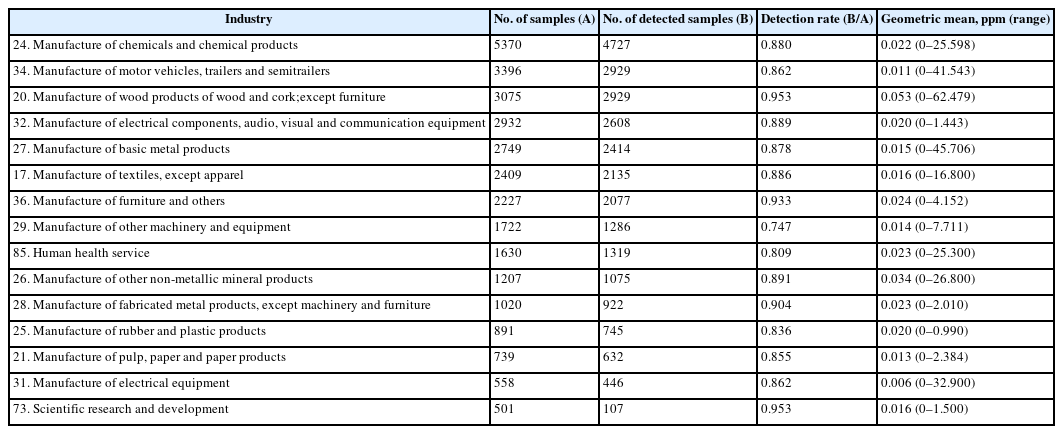
Top 15 number of samples of measurement of concentration of work-environmental formaldehyde by sub-categories of industries in Korea, 2002–2010
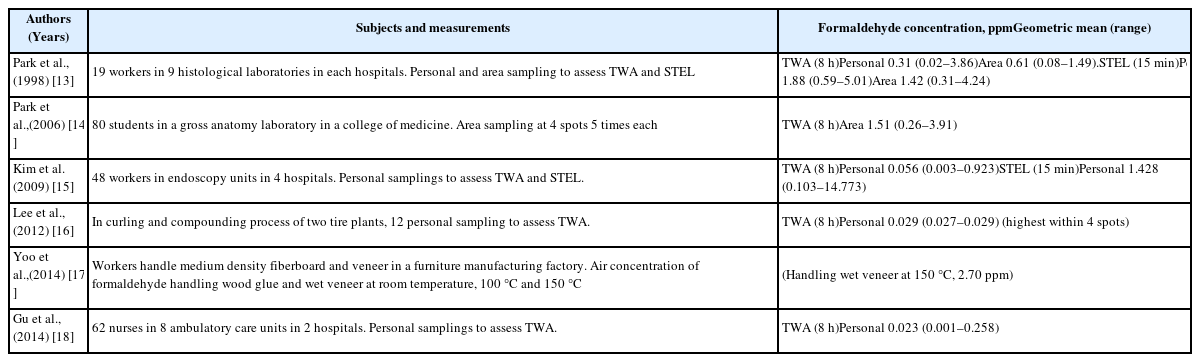
Table 3 shows the results of the formaldehyde exposure levels based on results from academic reports in Korea. The maximum exposure level was 5.01 ppm in histological laboratories from nine general hospitals [13]. The maximum formaldehyde exposure level was 3.91 ppm among 80 students in a gross anatomy laboratory, which was sampled five times in four areas [14]. The maximum formaldehyde exposure level was 14.77 ppm among 48 workers in the endoscopy units of four general hospitals [15].
The highest concentration of formaldehyde was 0.029 ppm in the compounding process of two tire manufacturing plants [16]. A furniture manufacturing factory had a formaldehyde concentration of 2.7 ppm when handling wet veneer at 150 °C [17]. The maximum formaldehyde exposure level was 0.258 ppm among 62 nurses in two university hospitals [18].
Epidemiological studies
NPC
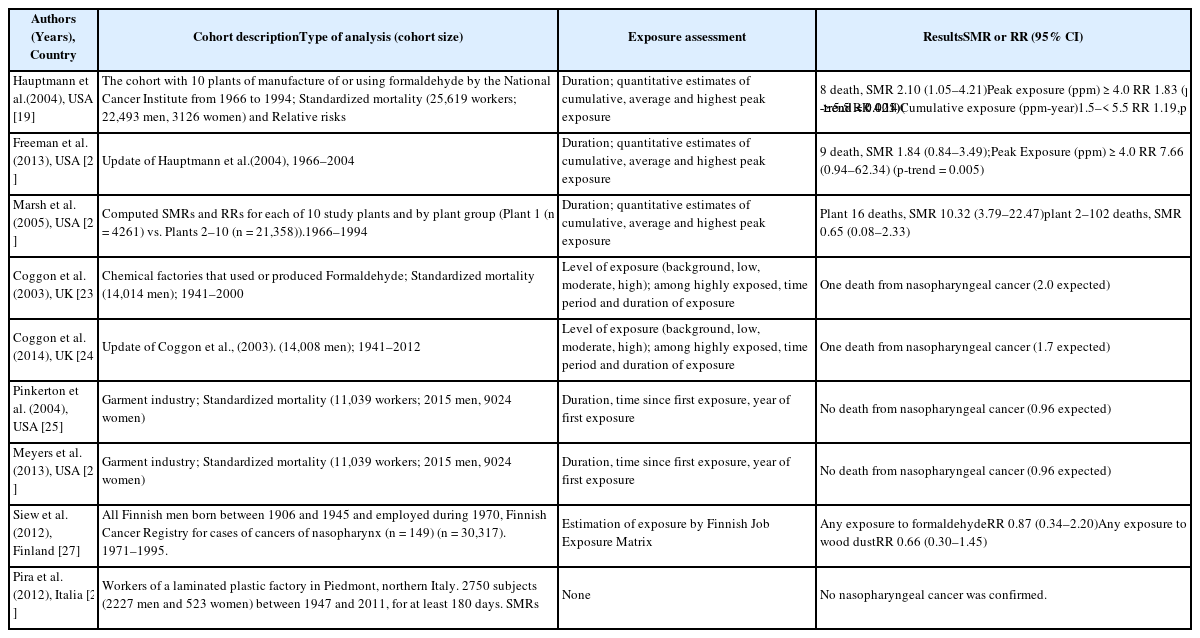
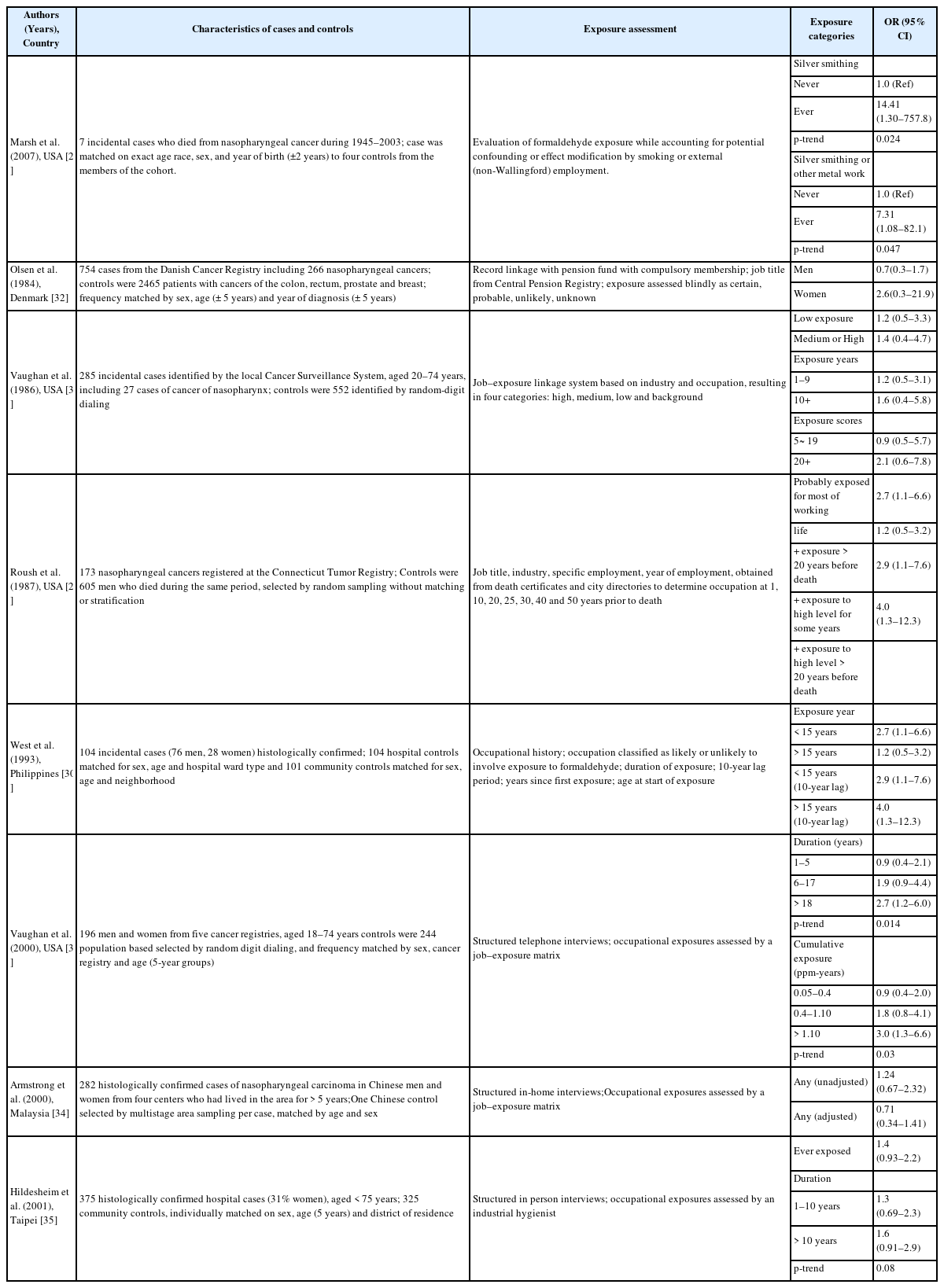
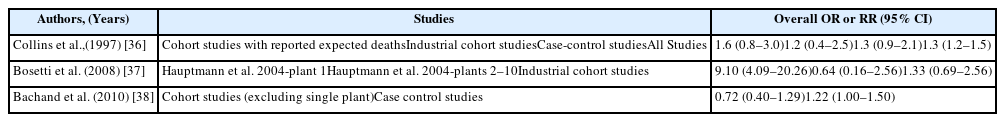
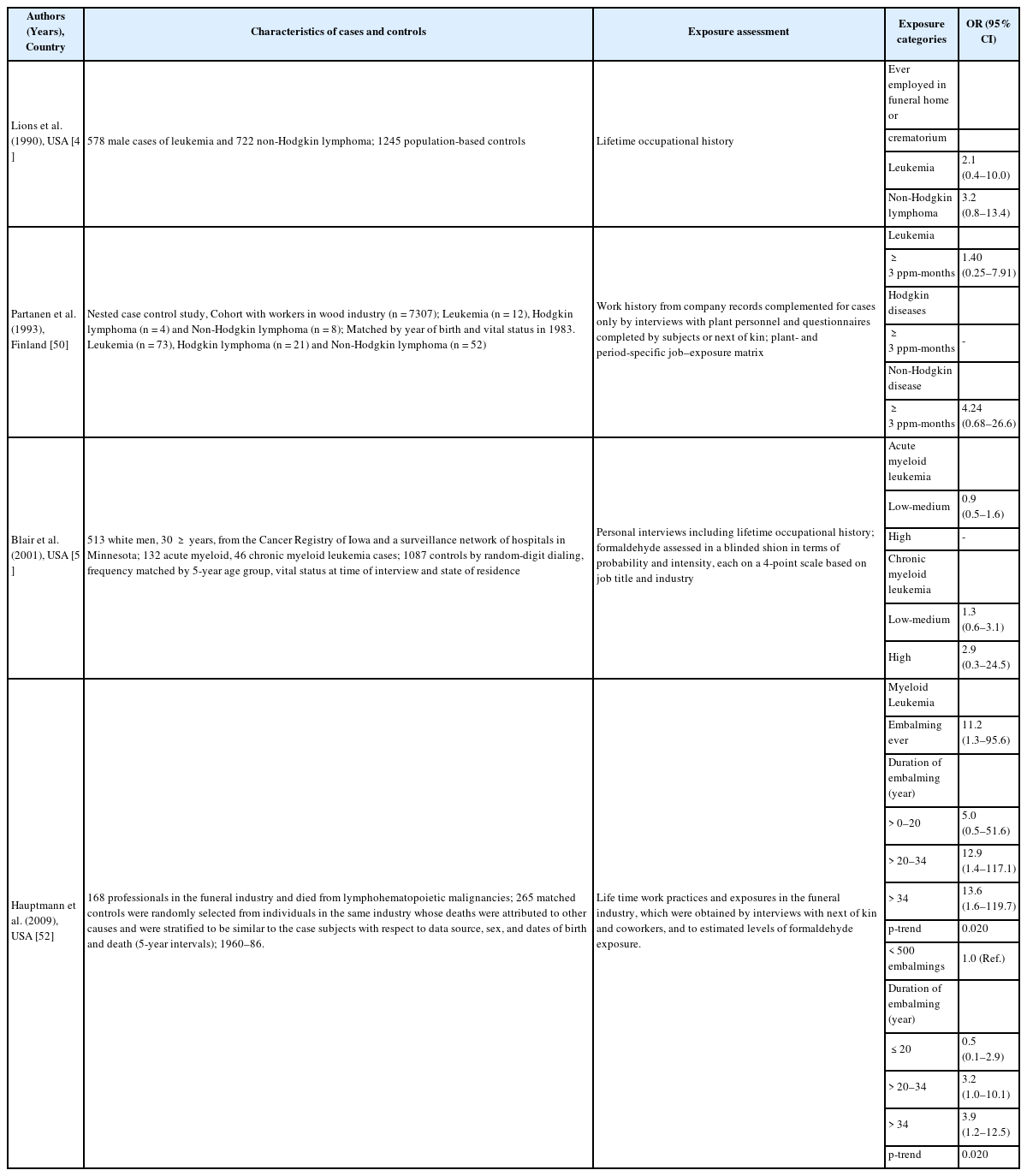
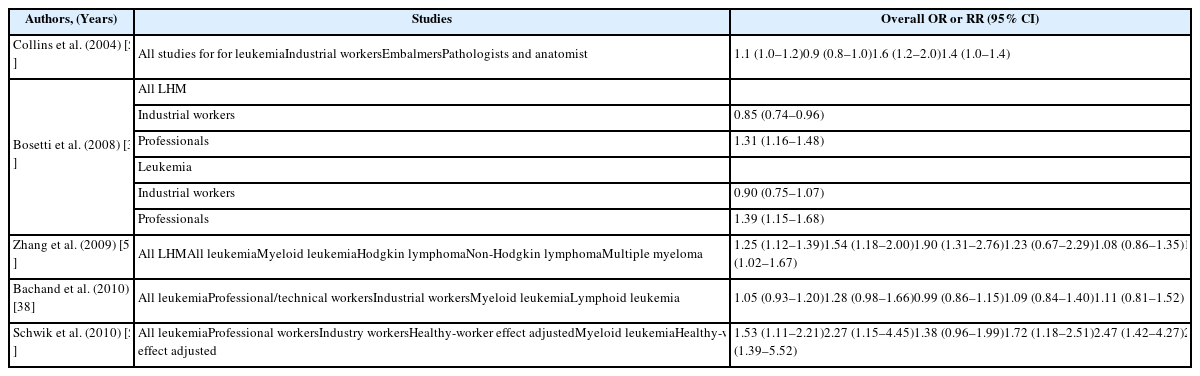
The main epidemiological results for NPC have been obtained from a National Cancer Institute (NCI) cohort that included 10 plants that produced or used formaldehyde. The results revealed a significantly increase risk of death because of NPC and dose-response relationships with both peak and cumulative formaldehyde exposures [19]. The strength of the associations weakened and the dose-response relationships for cumulative exposure levels disappeared after 10 years of follow-up [20]. Marsh et al. reported that this result was related to the effect of the first factory, and they reported that the excess death because of NPC was the result of a work history involving silversmithing or other metal processing [21, 22]. However, the IARC committee concluded that the effect modification based on silversmithing or other confounding could not explain the excess death because of NPC [4]. Another cohort study of a British chemical plant, an American clothing manufacturer, a Finish cancer registry, and an Italian plastic factory did not detect a significant risk of formaldehyde-related NPC, with the exception of an unexplained cluster of deaths because of NPC at plant 1 in the NCI cohort [23–28] (Table 4). Several case-control studies have also reported a significant relationship or dose-response relationship between the highest formaldehyde exposure and NPC [29–31]. However, we did not detect significant relationships in other studies [32–35] (Table 5). The results from a meta-analysis (excluding plant 1 of the NCI cohort) are shown in Table 6, and the meta-risk was 0.72 (95% confidence interval [CI]: 0.40–1.29) [36–38].
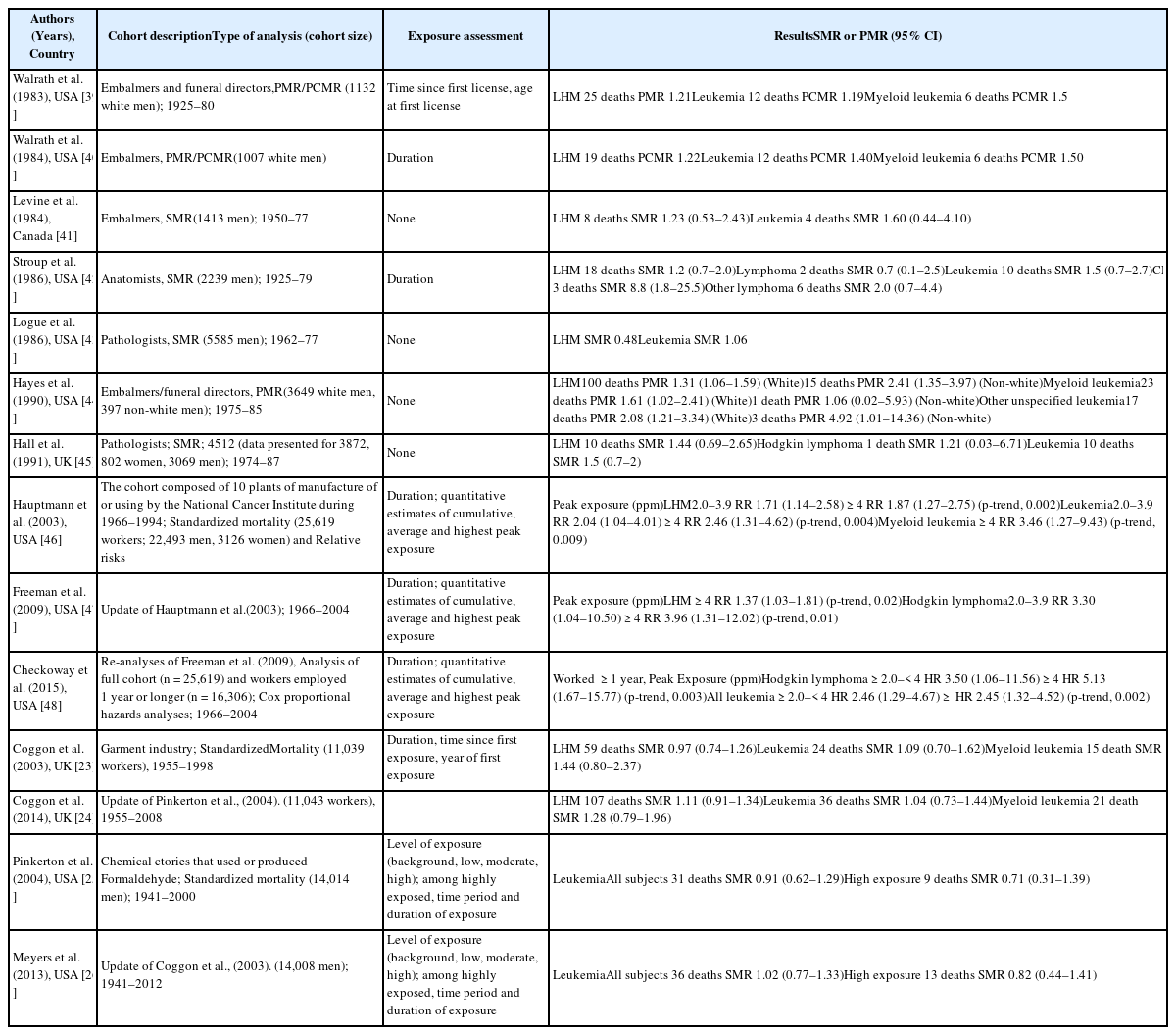
Lymphohematopoietic malignancies
Six of the seven mortality studies involving professional workers (e.g., embalmers, funeral directors, pathologists, and anatomists) revealed positive associations between formaldehyde exposure and lymphohematopoietic malignancies (LHM) [39–45] (Table 7). The NCI cohort compared deaths from 2004 and 1994, and found that the strength of association between formaldehyde exposure and death because of leukemia and myeloid leukemia was weakened. Furthermore, the peak-exposure category (≥4.0 ppm) exhibited dose-response relationships with LHM, myeloid leukemia, and Hodgkin lymphoma [46–48]. Three cohort studies failed to detect a significantly increased risk of death [23–26]. Three case-control studies of formaldehyde exposure and leukemia also failed to detect a significantly increased risk [49–51]. A nested case-control study of funeral professionals revealed that the risks of non-lymphoid LHM or myeloid leukemia increased with working experience [52] (Table 8). Table 9 shows results from a meta-analysis of the relationship between formaldehyde exposure and leukemia [37, 38, 53–55]. The risk estimate for all leukemia was 1.05 (95% CI: 0.93–1.20) when researchers included the recent NCI cohort and excluded proportionate mortality studies [38]. The meta-risks including the NCI cohort and American funeral industries were 1.53 (95% CI: 1.11–2.21) for all leukemia and 2.47 (95% CI:1.42–4.27) for myeloid leukemia [55]. Nevertheless, researchers have not reached a consensus regarding any causal association or dose-response relationship between formaldehyde exposure and LHM, including myeloid leukemia [56, 57]. However, there appears to be a causal association between formaldehyde exposure, and especially peak exposures of ≥4 ppm, and all LHM (including Hodgkin lymphoma but not leukemia).
Biological plausibility
There is no clear carcinogenic mechanism regarding formaldehyde exposure and NPC or LHM. However, formaldehyde exposure can lead to the formation of DNA-protein crosslinks in vitro, as well as genotoxicity in human nasal cells and disruption of bone marrow stem cells, hematopoietic stem cells, circulating progenitor cells, and primitive pluripotent stem cells [58, 59]. Chromosomal aneuploidy in circulating myeloid progenitor cells has also been identified among healthy workers who were exposed to formaldehyde [60].
Criteria for recognizing formaldehyde as an occupational carcinogen
The IARC classified formaldehyde as a definite human carcinogen (Group 1) for NPC in 2004 and leukemia (especially myeloid leukemia) in 2012. Formaldehyde was also classified as a suspected human carcinogen (Group 2A) for sino-nasal cancer in 2012 [3, 4]. The American National Toxicology Program (NTP) also classified formaldehyde as a ‘known human carcinogen’ in 2011 [61]. Furthermore, the EU CLP classified formaldehyde as a class 1B carcinogen, which indicates that the substance has presumed carcinogenic potential in humans, based on experimental animal data [6].
The ILO includes 20 carcinogens on its list of occupational cancers, although it does not define the related cancers. The tripartite commission of ILO included formaldehyde on its list of potential carcinogens, although formaldehyde was not included in the final list in 2009, as employers demanded a deeper review of the data [8, 9]. South Korea, France, Denmark, Malaysia, and Taiwan have clearly recognized the relationship between occupational cancer and formaldehyde exposure [9–11]. France also recognized that NPC could be caused by exposure to formaldehyde or its polymers in 2009 [10]. However, the list of recognized occupational diseases in Finland does not include formaldehyde-related cancers, although it was considered in the “Memorandum from the Occupational Cancer Working Group 2013” [62]. Moreover, the EU only recognizes a relationship between formaldehyde exposure and NPC, as there is insufficient epidemiological evidence regarding LHM [63].
Compensation cases and considerations for approval
South Korea has not compensated any cases that were related to formaldehyde exposure itself, although some cases have been compensated after mixed exposures to other chemicals. In 2012, a 61-year-old man developed multiple myeloma after working at a poultry farm for 16 years and being exposed to agricultural chemicals (pesticides and/or organic solvents, such as formaldehyde), with an average estimated formaldehyde exposure level of 17.53 ppm [64]. A 43-year-old man was diagnosed with myelodysplastic syndrome after working in a furniture manufacturing factory for 22 years. In 2013, the man’s tasks involved cutting and fabricating medium-density fiberboard, as well as pasting and polishing veneer. He was exposed to benzene and formaldehyde (a TWA concentration of 0.312 ppm/8 h), which corresponded to a cumulative level of 6962–10,016 ppm·hour, and a cumulative benzene exposure of 1.88–11.25 ppm·year [65].
Recognition criteria and consideration issues
Since 2013, the occupational disease criteria of the Enforcement Decree Industrial Accidents Compensation Insurance Act has included “leukemia or NPC caused by formaldehyde exposure” [2]. However, there is little evidence regarding the cumulative exposure level, minimum exposure duration, extent of exposure, and combined exposure or latent period. The results from the NCI cohort and the World Trade Center Health Program suggest latent periods of approximately 15 years for NPC and 2 years for LHM, based on statistical modeling and epidemiological studies [19, 46, 66]. In addition, the EU’s “Information notices on occupational diseases: a guide to diagnosis” suggest a 10-year latent period for NPC and 6 months for the minimum exposure duration, despite the absence of definitive scientific evidence [63]. The results from NCI cohort studies also suggest that peak exposures of ≥4.0 ppm were important for LHM and Hodgkin lymphoma [20, 47]. Finally, there is a considerable risk of combined formaldehyde exposure, as the known environmental risk factors for NPC include Epstein-Barr virus infection, consuming salted fish and reserved food spicy food, chronic ear-nose-and-throat conditions, and occupational exposures (e.g., wood dust, industrial heat or combustion products, cotton dust, and solvents, such as phenoxy acid and chlorophenol). These factors must also be considered when determining whether cases are eligible for compensation [67, 68]. Moreover, exposure to benzene, 1,3-butadiene, or ethylene oxide is also an important risk for LHM [69].
Discussion
The IARC and NTP have classified formaldehyde as a definite human carcinogen, although the US EPA, ACGIH, and EU CLP disagree with this classification [4–7]. A few countries, including South Korea, have also listed formaldehyde as an occupational carcinogen [2, 9–11] because of the relatively low risks of NPC or LHM in meta-analyses and cohort studies (vs. other occupational cancers). Furthermore, it is difficult to quantify FORMALDEHYDE exposure and NPC has a very low incidence (approximately 1/100,000 population) [70]. However, there is sufficient epidemiological evidence to confirm associations with LHM and Hodgkin lymphoma, especially in terms of peak exposure, based on a recent update of the NCI cohort, three recent meta-analyses, and a nested case-control study of embalmers [4, 47].
In South Korea, the peak exposure in various industries was 2.70–14.8 ppm, and the TWA exposure level was 1.0–62.5 ppm in work-environment measurements. Thus, the risk of NPC or LHM could be increased among South Korean pathologists, anatomy students, and furniture workers with a peak exposure of ≥4 ppm [13–15, 17]. In most regions, the age-standardized incidence of NPC among men and women is < 1/100,000 person-years [70]. However, dramatically elevated rates are observed in the Cantonese population of southern China (including Hong Kong) [68]. These regional differences may be related to environmental risk factors, such as Epstein-Barr virus infection, and/or diet [67]. Thus, we suggest that both occupational exposure and environmental risk factors should be considered in the process of approving LHM cases for workers’ compensation.
The present study provided a review of the recent epidemiological evidence regarding the relationships between formaldehyde exposure and NPC or LHM, as well as a discussion regarding factors that could influence the recognition of formaldehyde-related cancers as occupational cancers. However, there is insufficient data regarding peak exposure levels and average exposure levels in various South Korean industries and jobs. Thus, additional studies are needed to help develop compensation policy and achieve scientific consensus.
Conclusion
We identified causal relationships and significant dose-response relationships between formaldehyde exposure and NPC, all LHM, and Hodgkin lymphoma. Furthermore, it appears that peak exposure is the most relevant factor when considering whether to officially recognize formaldehyde-related occupational cancers. Therefore, it is important to control formaldehyde exposure to protect workers and prevent them from developing NPC or LHM.
Abbreviations
ACGIH
American Conference of Governmental Industrial Hygienists
EPA
Environmental Protection Agency
EU CLP
European Union Occupational Disease Classification, Labelling and Packaging of Substances and Mixtures
IARC
International Agency for Research on Cancer
ILO
International Labor Organization
LHM
lymphohematopoietic malignancies
NCI
National Cancer Institute
NPC
nasopharyngeal cancer
NTP
National Toxicology Program
TLV-TWA
threshold limit value-time weighted average
Acknowledgements
None
Funding
This work was supported by Ministry of Employment and Labor.
Availability of data and materials
Not applicable
Authors’ contributions
SCK drafted the article. SCK, JS, and JP searched and assisted the related references. IK and JS supported and advised medical view. All of the authors read and approved the final manuscript.
Notes
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.