Recognized cases of amyotrophic lateral sclerosis in automobile workers by the Korean Epidemiologic Investigation Evaluation Committee
Article information
Abstract
Background
Three automobile company workers (one from Factory D and two from Factory E) were diagnosed with amyotrophic lateral sclerosis. The Korean Epidemiologic Investigation and Evaluation Committee determined that there is considerable scientific evidence supporting the association between amyotrophic lateral sclerosis and combined exposure to heavy metals, organic solvents, and diesel exhaust at the manufacturing plant.
Case presentation
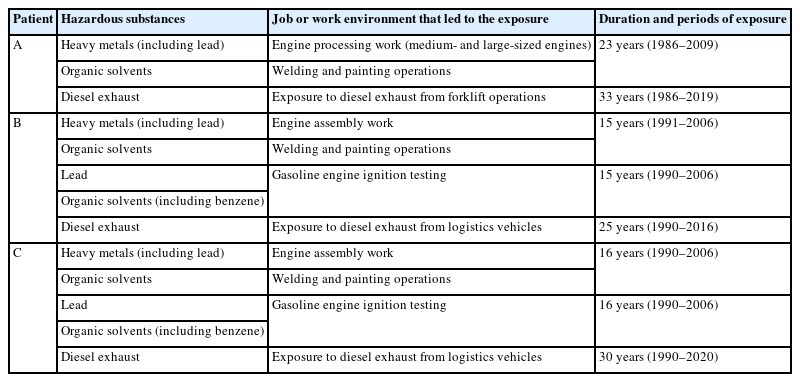
Patient A, who primarily engaged in engine processing and completed vehicle inspection at Factory D, was exposed to considerable amounts of heavy metals and organic solvents during medium- and large-engine processing, welding, and painting for over 23 years. Additionally, the patient was likely exposed to diesel exhaust for 33 years from forklifts delivering engines in the workshop. Patients B and C, who were responsible for engine assembly, ignition testing, and engine shipment at Factory E since around 1990, were exposed to lead and benzene from gasoline during engine ignition tests in the engine department for 15 and 16 years, respectively. They also encountered welding fumes, heavy metals, and organic solvents during welding and painting tasks. In addition, Patients B and C were continuously exposed to diesel exhaust from logistics vehicles on standby during work hours for 25 and 30 years, respectively.
Conclusions
Although the specific level of lead exposure causing amyotrophic lateral sclerosis remains undetermined, numerous studies have consistently reported a relationship between lead exposure and disease development. Limited evidence suggests that exposure to organic solvents and diesel exhaust may increase the risk of amyotrophic lateral sclerosis. Therefore, the Epidemiological Investigation and Evaluation Committee concluded that the three patients’ work-related exposure to heavy metals, organic solvents, and diesel exhaust is significantly supported by scientific evidence as a cause of their amyotrophic lateral sclerosis.
BACKGROUND
The incidence of amyotrophic lateral sclerosis (ALS) in Europe and North America is estimated to range from 1.55 to 2.7 per 100,000 individuals, with a prevalence of 2.7 to 7.4 per 100,000 people. Previous reports have suggested that its incidence and prevalence are lower among Africans, Asians, and Hispanics than among Caucasian populations. However, whether specific racial groups have inherently lower incidence or prevalence rates cannot be definitively determined due to the various research methodologies used across countries. The prevalence ratio between men and women is approximately 1.3–1.5 to 1, indicating a higher rate in men. Nonetheless, this sex disparity almost disappears after the age of 70 years. The onset of ALS tends to increase with age, particularly after 40 years, peaking at age 74 years, and then declining.1-3
ALS is classified into familial and sporadic types, with sporadic cases lacking specific genetic risk factors and accounting for 90%–95% of all cases. Most familial ALS cases are inherited in an autosomal dominant manner, with about 20% caused by mutations in chromosome 21, which houses the gene responsible for encoding the enzyme superoxide dismutase 1.
ALS is a multifactorial and multifocal disease with an unclear pathogenesis. Various potential causes, including genetic factors, autoimmune reactions, occupational and environmental influences, oxidative stress, glutamate excitotoxicity, mitochondrial damage, defective axonal transport, and abnormalities in glial cells, are being investigated. In addition, metabolic abnormalities in DNA and RNA, paraneoplastic syndromes, lymphomas, plasma cell disorders (paraproteinemia), toxic effects of heavy metals, and viral infections are also being explored. However, the precise pathogenesis of ALS is yet to be explained.4
Patients with ALS typically present with progressive voluntary muscle weakness that spreads to adjacent body segments, often succumbing to respiratory failure within 2–4 years of diagnosis. The main pathological findings include cytoplasmic inclusion bodies, eosinophilic Bunina bodies, and ubiquitinated TAR DNA-binding protein 43 (TDP-43). Up to 60% of cases are accompanied by cognitive and behavioral changes, with 15% developing frontotemporal dementia due to secondary brain structure changes.5 There is no definitive test for ALS; diagnosis relies on identifying progressive signs in both upper and lower motor nerves, electromyography findings, and ruling out other diseases with similar symptoms.6
Recognized risk factors for ALS include age, family history, and smoking.7-9 Evidence suggests that individuals in occupations with potential exposure to harmful substances, such as heavy metals, organic solvents, and pesticides, have a higher risk of developing ALS.10-12 Specifically, an increased risk of ALS has been reported with exposure to heavy metals (especially lead),13,14 organic solvents,15-18 and diesel exhaust.19-21
CASE PRESENTATION
Patient A
Patient information and medical history
The patient was a 55-year-old male. Patient A developed weakness in the right leg in February 2019 and symptoms of weakness in the right arm and fasciculation in both arms and legs in August 2019. He tripped over an object while working and received treatment for his right gluteus medius. In October 2019, electromyography revealed abnormal findings, prompting a visit to a university hospital on October 17, 2019, where sporadic ALS was diagnosed.
Social history, family history, and past history
Patient A had quit smoking 20 years ago, with a 2.5-pack-year smoking history before quitting. He quit drinking in 2018, prior to which he drank alcohol about once a month, consuming 1–2 glasses of beer each time. He had no family history of diseases related to ALS.
Occupational history
Patient A worked in an automobile factory. He performed medium- and large-engine processing work from 1986 to 2009 (about 23 years) and inspection work on completed vehicles from 2009 to 2019 (about 10 years).
Exposure assessment
The patient processed medium- and large-sized engine materials for about 23 years. Preliminary processing involved cutting blocks of the cast material, secondary processing involved using cutting oil, and precision processing involved using honing oil. After processing, the metal dust was washed away at high pressure with cleaning oil at temperatures >80°C. To prevent rust, the cleaned engines were immersed in an impregnation tank using a hoist and then removed and placed on a pallet. The patient was continuously exposed to organic solvents contained in cutting oil, cleaning oil, and rust-preventive agents used during engine processing.
Until 1998, the patient also performed additional tasks, including welding 1–3 days a week for an average of 3 hours per session. He used gloss paint diluted with thinner for painting during overtime hours. According to the 2014 research report of the Occupational Safety and Health Research Institute on blood lead concentration by industry from 2000 to 2013, welding is associated with a high risk of lead poisoning. In particular, individuals involved in the burning of lead or lead-coated substances were considered a high-risk work group for lead exposure. According the report, the average lead level in the blood of workers in the automobile manufacturing industry ranged from 3.17 to 4.846 μg/dL, with a maximum value of 170.1 μg/dL.22
While the patient worked in the engine processing department, a diesel forklift entered the workplace to deliver engine materials and transport processed engines more than 40 times a day. A 2002 work environment measurement revealed that nitrogen dioxide levels were 0.3584 ppm, which exceeded the American Conference of Governmental Industrial Hygienists (ACGIH) standard (time weighted average 0.2 ppm).
Since the inspection department also utilized diesel trucks, the patient was likely continuously exposed to diesel exhaust until his departure from the company. The inspection department's 2016 work environment measurement results indicated that nitrogen dioxide levels were 0.6528 ppm, which was more than three times the ACGIH exposure standard.
Patient B
Patient information and medical history
The patient was a 49-year-old male. Patient B began experiencing right shoulder pain in early 2015. Although the pain improved, muscle weakness in the right arm gradually worsened over 5–6 months. Therefore, he was admitted to a general hospital in June 2015, where electromyography confirmed the presence of right brachial plexopathy. In August 2015, he reported fasciculation of the right biceps brachii. Starting in November 2015, similar symptoms appeared in the left upper arm, with bilateral upper arm tremors and weakness intensifying. The patient reported muscle tremors in both knees in December 2015. On April 11, 2016, he visited a general hospital, and sporadic ALS was diagnosed based on electromyography.
Social history, family history, and past history
Patient B quit smoking after 2014, but the duration and quantity of smoking before he quit could not be confirmed. He reported consuming 1–2 bottles of soju weekly before the illness but stopped drinking after becoming ill. His smoking and drinking histories were not documented in his medical records. There was no notable disease history or family history of diseases related to ALS.
Occupational history
Patient B began working at an automobile factory in 1988 and was involved in engine assembly, engine ignition testing, and logistics work for engine shipment using a forklift for approximately 27 years until his diagnosis in 2016. He carried out engine inspection work from 1988 to 1991 (about 2 years) and focused on engine ignition testing, engine assembly, and engine shipment work from 1991 to 2006 (about 15 years). After a process transfer, he was tasked with engine assembly, forklift operation, and facility management, excluding work in the engine test room, from 2006 to 2016 (about 10 years).
Exposure assessment
Patient B was likely exposed to lead and aromatic hydrocarbons (benzene, toluene, xylene, etc.) contained in the gasoline used as fuel, among the combustion substances generated during the engine ignition test. The Ministry of Power and Energy of the Republic of Korea revised the Enforcement Rules of the Petroleum Business Act on November 7, 1986, setting the standard for lead content in unleaded gasoline to ≤0.013 g/L. To increase the octane rating of unleaded gasoline, the content of aromatic hydrocarbons such as benzene, toluene, and xylene was increased compared to that in leaded gasoline. It is presumed that unleaded gasoline was utilized during the engine ignition tests performed by the patient. A 1991 work environment measurement revealed that lead was present at 0.00583 mg/m³, which is approximately 12% of the 2023 exposure standard (0.05 mg/m³).
Until the early 2000s, the patient undertook additional tasks, such as process facility repairs, rust removal from old pallets, spray painting, and welding, twice a month to improve the working environment. Work environment measurements in 2005 and 2006 recorded welding fume levels of 8.4357 mg/m³ and 8.1399 mg/m³, respectively, which exceeded the Ministry of Employment and Labor of the Republic of Korea's exposure standard (5 mg/m³). During this additional work, the patient painted 300–400 pallets annually with gloss paint, likely exposing him to welding fumes, heavy metals, and organic solvents in the paint.
The patient reported that he did not directly handle metalworking fluid but was indirectly exposed to it from a distance of approximately 5 meters away. A 2002 study that assessed exposure to polynuclear aromatic hydrocarbons (PAHs) in workers handling metalworking fluids found that 91 of 120 airborne PAH samples (75.8%) were above the detection limit.23 Exposure levels for heat treatment and non-heat treatment workers were 3.44 μg/m³ and 0.13 μg/m³, respectively. A National Institute for Occupational Safety and Health report on exposure to metal processing fluids in automobile manufacturing plants recorded formaldehyde levels ranging from 0.041 to 0.190 ppm in 16 individual samples.24
Workers reported that diesel engine emissions from logistics vehicles constantly idling while loading and unloading engines flowed into the factory. A 2016 work environment measurement found that nitrogen dioxide levels were 0.09121 ppm, which was 45% of the ACGIH exposure standard.
Patient C
Patient information and history of presenting illness
The patient was a 50-year-old male. Patient C began experiencing stiffness in both calves in September 2019. By November 2019, he noticed that his right foot felt heavy and dragged when walking on flat surfaces. He observed a tendency to lean to the right while walking since December 2019. Brain and spine magnetic resonance imaging (MRI) and electromyography revealed motor nerve damage. In January 2020, the patient experienced leg stiffness and developed calf cramps while walking. Cervical spine and lumbosacral MRI tests revealed no significant abnormalities. In February 2020, he was admitted to a tertiary general hospital because of persistent symptoms and speech disturbances. On February 27, 2020, sporadic ALS was diagnosed using electromyography.
Social history, family history, and past history
The patient stopped smoking after 2000, but the quantity he smoked before quitting was not specified. He did not consume alcohol. His medical history included benign prostatic hyperplasia and dyslipidemia. He had no family history of diseases related to ALS.
Occupational history
The patient was employed at an automobile factory since 1990, where he was involved in engine assembly, engine ignition testing, and engine shipment (including forklift operations) logistics work for approximately 30 years until his diagnosis in 2020. Between 1990 and 2006 (about 16 years), his responsibilities included engine ignition testing, engine assembly, and engine shipping. Between 2006 and 2020 (about 14 years after a process transfer), he performed tasks such as engine assembly, forklift operation, and facility management, with the exception of working in the engine test room.
Exposure assessment
Since 1990, the patient engaged in the same processes as Patient B; therefore, the evaluation of Patient C’s exposure has been omitted. There were no differences in exposure history between the two patients, except for the variance in the working period due to the different timings of ALS onset.
Ethics statement
This study was approved by the Institutional Review Board (IRB) of the Occupational Safety and Health Research Institute (IRB No. OSHRI-202303-HR-013), and written informed consent for the publication of this report and any accompanying data was obtained from the patients or their families.
DISCUSSION AND CONCLUSION
While working in the engine construction department for approximately 23 years, Patient A was exposed to significant amounts of heavy metals (including lead) and organic solvents. Additionally, during his 33 years in the engine construction and assembly department, he was exposed to substantial amounts of diesel exhaust gas. Insufficient use of exhaust devices and protective equipment during work likely led to prolonged exposure to various harmful substances. Patients B and C, who worked in the engine department for about 25 and 30 years, respectively, were exposed to lead and benzene from gasoline during engine ignition tests for approximately 15 and 16 years, respectively. They were also likely to have been exposed to welding fumes, heavy metals, and organic solvents during welding and painting operations. Furthermore, they were continuously exposed to diesel exhaust gas emitted from logistics vehicles idling nearby. The job-related exposures of patients A, B, and C are summarized in Table 1.
Patients A, B, and C developed ALS at the ages of 55, 49, and 50 years, respectively, which is earlier than the peak age for ALS onset, and none had a medical or family history related to neurological diseases. Despite a genetic predisposition, clear occupational hazards associated with ALS have not been definitively identified. This ambiguity may stem from the difficulty of statistical interpretation due to the very low prevalence of the disease (2.7–7.4 per 100,000 persons). Despite the very low prevalence, the reported cases developed the same disease through the same or similar processes. Although quantitatively assessing patients' exposure to harmful substances is challenging, the epidemiological investigation committee which is described in Table 2 concluded that the patients had been chronically exposed to lead, organic solvents, and diesel exhaust gas during their work.
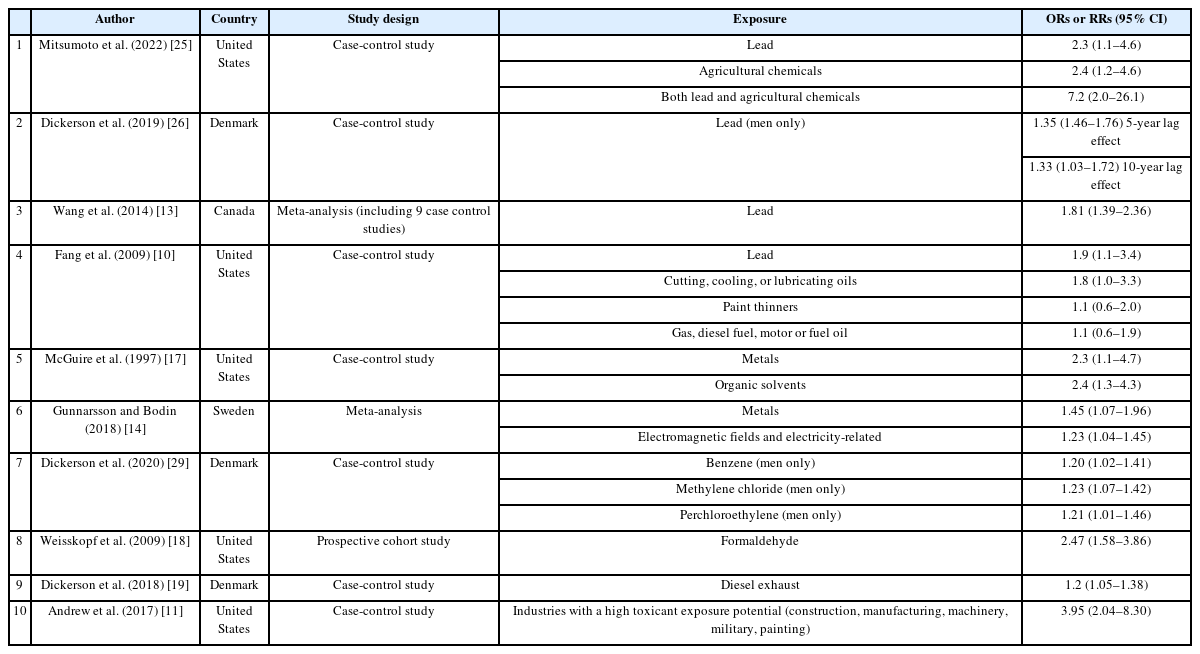
Considering the following epidemiologic studies (described in Table 3), the committe concluded that there was a high occupational association with complex exposure in the cases of Patients A, B, and C in 2022 and 2023.
Summary of published studies related to occupational exposure to organic solvents, heavy metals (especially lead) and diesel exhaust related to ALS
A 2009 UK case-control study reported that self-reported exposure to paint removers, cutting oils, coolants, and lubricants increased the risk of developing ALS by 60%–90%. Chemicals that increased the risk of ALS by >50% included aliphatic chlorinated hydrocarbons, glycols, glycol ethers, and hexane.10 In a 2007 case-control study, exposure to one or more chemicals (pesticides, organic solvents, and heavy metals) related to occupation or hobbies increased the risk of developing ALS (odds ratio [OR]: 2.51; 95% confidence interval [CI]: 1.64–3.89). Workers in industries with a high likelihood of exposure to toxic substances (construction, manufacturing, machinery industry, military, painting) had an increased risk of developing ALS (OR: 3.95; 95% CI: 2.04–8.30).11 A 2022 US case-control study using the national ALS registry found that patients with ALS were more likely to report occupations exposed to agricultural chemicals (180 potential neurotoxicants, including pesticides and herbicides) or lead. The risk was greater for occupations exposed to both lead and agricultural chemicals (OR: 7.2; 95% CI: 2.0–26.1) than for those exposed to lead alone (OR: 2.4; 95% CI: 2.2–4.6) or agricultural chemicals alone (OR: 2.2; 95% CI: 2.0–26.1).25
In a 2019 Danish population-based cohort study, applying a lag time of 5 years to men with a ≥50% probability of lead exposure (using a Job Exposure Matrix [JEM]) revealed that the cumulative estimated exposure had an OR of 1.35 (95% CI: 1.46–1.76) in the high exposure group (>222 µmol/L) above the 60th percentile. When a 10-year lag effect was considered, the OR was 1.33 (95% CI: 1.03–1.72). However, these findings were not statistically significant in the overall cohort or in the female lead exposure group.26 A subsequent 2020 Danish population-based cohort study assessed occupational exposure to chromium, iron, and nickel among patients with ALS and controls. The study suggested a potential increase in ALS risk among men exposed to chromium and women exposed to nickel, though neither finding was statistically significant or demonstrated a strong correlation.27 Nonetheless, a meta-analysis of nine case-control studies on lead exposure (OR: 1.81; 95% CI: 1.39–2.36) and four case-control studies on heavy metal exposure (OR: 1.87; 95% CI: 1.51–2.33) indicated that past occupational exposure to lead and heavy metals was associated with an increased incidence of ALS.13
According to a 1992 study, a very high OR (OR: 15.6; 95% CI: 2.8-87.0) was observed when three risk factors—heritability, organic solvent exposure, and being male—were considered together in a case-control study examining the association between motor neuron disorders and occupational exposure. However, a limitation was noted due to the wide CI and the small sample size of only seven patient groups with this combination of factors.15 A 1993 case-control study utilizing Scottish Motor Neuron Disease Registry data indicated that exposure to chemicals/organic solvents increased the risk of motor neuron disease (OR: 3.3; 95% CI: 1.3–10), but the wide CI and lack of replication in subsequent studies were noted as limitations.16,28 In 1997, a case-control study of 174 patients with ALS sought to identify the correlation between workplace hazards (metals, organic solvents, pesticides, etc.) and ALS. It found that self-reported exposure to organic solvents increased the OR to 2.4 (OR: 2.4; 95% CI: 1.3–4.3).17 A 2009 case-control study investigating the association between ALS, occupational history, and detailed exposure factors (solvents, formaldehyde, lead, etc.) demonstrated that the risk of developing ALS was 60%–90% higher in cases of exposure to paint remover, cutting oil, cooling oil, and lubricant; antifreeze or coolant, and dry cleaner. Chemicals that increased the risk of ALS by more than 50% included aliphatic chloride hydrocarbons, glycols, glycol ethers, and hexane, and the relative risk associated with these occupational exposures was greater among non-smokers.10 A large prospective study in 2009 evaluated the exposure to 11 substances (including pesticides, asbestos, and organic solvents) and the risk of death from ALS. For formaldehyde exposure, the risk ratio was significantly higher at 2.47 (relative risk: 2.47; 95% CI: 1.58–3.86), showing a dose-response relationship with exposure duration. However, no other epidemiological evidence supports the association between formaldehyde exposure and ALS occurrence, and caution in interpreting this finding is needed due to the self-report method used for exposure measurement.18
A review paper summarizing studies published before 2018 reported that excessive physical work (e.g., professional athletes), exposure to metals (primarily lead), and exposure to chemicals (primarily pesticides) increased the relative risk of developing ALS. Additionally, the relative risk of ALS was found to be higher for individuals exposed to electromagnetic fields or those working in electricity-related occupations, such as nurses and doctors.14 A 2020 Danish population-based cohort study estimated exposure through a JEM and confirmed the association of exposure to benzene (OR: 1.20; 95% CI: 1.02–1.41), methylene chloride (OR: 1.23; 95% CI: 1.07–1.42), and perchloroethylene (OR: 1.21; 95% CI: 1.01–1.46) in men. However, this study did not find a significant association with exposure to organic solvents in women, suggesting potential sex-specific characteristics.29
Several occupational epidemiological studies, aside from some literature, have demonstrated that the risk of ALS increases for workers in jobs exposed to diesel exhaust gas (e.g., truck drivers, construction workers, machine operators, bus drivers, and soldiers). A 2018 case-control study found an association between diesel exhaust gas exposure and the risk of ALS in men (OR: 1.2; 95% CI: 1.05–1.38).19 A 2022 review paper summarizing research on the link between military service and the onset of ALS indicated that the risk of ALS may increase due to environmental exposure (e.g., diesel exhaust gas, jet exhaust, brake/tire wear powder, incinerators) encountered during military service.20 A 2023 study on the association between urbanization, air pollution, and water pollution and the occurrence of ALS suggested that nitrogen dioxide, a major product of diesel exhaust and diesel combustion, was associated with an increased risk of ALS.21
In conclusion, the three cases show that automobile company workers can be exposed to lead, organic solvents, and diesel exhaust, which may increase the risk of ALS. Workers should mitigate their exposure to hazardous substances in the workplace.
Abbreviations
ACGIH
American Conference of Governmental Industrial Hygienists
ALS
amyotrophic lateral sclerosis
CI
confidence interval
JEM
Job Exposure Matrix
MRI
magnetic resonance imaging
OR
odds ratio
PAH
polynuclear aromatic hydrocarbon
TDP-43
TAR DNA-binding protein 43
Notes
Competing interests
The authors declare that they have no competing interests.
Author contributions
Conceptualization: Ye S. Data curation: Kim Y. Investigation: Kim Y, Ye S, O JH, Cho H. Writing - original draft: Kim Y. Writing - review & editing: Kim Y, Ye S.
Acknowledgments
The authors thank the Occupational Safety and Health Research Institute and the Korean Occupational Safety and Health Agency for formally providing de-identified workers’ data.